How Many Electrons Does Sodium Have In Its Outer Shell
News Leon
Apr 01, 2025 · 6 min read
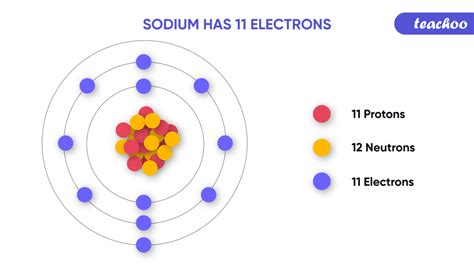
Table of Contents
How Many Electrons Does Sodium Have in Its Outer Shell? Understanding Sodium's Electronic Structure
Sodium (Na), a ubiquitous element found in table salt and crucial for biological processes, holds a fascinating position in the periodic table. Its reactivity and properties are intrinsically linked to its electronic structure, specifically the number of electrons residing in its outermost shell, also known as the valence shell. This article delves deep into understanding sodium's electronic configuration, explaining how many electrons it possesses in its valence shell and why this number is so significant in determining its chemical behavior.
Understanding Electron Shells and Valence Electrons
Before we delve into sodium's specifics, let's establish a foundational understanding of electron shells and valence electrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons are arranged in distinct energy levels or shells. The shells are numbered sequentially, starting with the shell closest to the nucleus (n=1), followed by n=2, n=3, and so on. Each shell can only hold a specific maximum number of electrons:
- Shell 1 (n=1): Maximum 2 electrons
- Shell 2 (n=2): Maximum 8 electrons
- Shell 3 (n=3): Maximum 18 electrons
- Shell 4 (n=4): Maximum 32 electrons
and so forth. The pattern follows the formula 2n², where 'n' is the shell number.
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound and, therefore, are the ones primarily involved in chemical bonding. The number of valence electrons dictates an atom's reactivity and the type of bonds it can form. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases with their filled outer shells (eight electrons, except for helium with two). This is the basis of the octet rule.
Sodium's Electronic Configuration and Valence Electrons
Sodium (Na) has an atomic number of 11, meaning it possesses 11 protons and, in its neutral state, 11 electrons. To understand its electron arrangement, we need to fill the electron shells according to the Aufbau principle, which states that electrons fill the lowest energy levels first. Therefore, sodium's electronic configuration is:
1s² 2s² 2p⁶ 3s¹
Let's break this down:
- 1s²: Two electrons fill the first shell (n=1).
- 2s² 2p⁶: Eight electrons fill the second shell (n=2): two in the 2s subshell and six in the 2p subshell.
- 3s¹: One electron occupies the third shell (n=3).
This reveals that sodium's outermost shell, the third shell (n=3), contains only one electron. Therefore, sodium has one valence electron.
Significance of Sodium's Single Valence Electron
The presence of a single valence electron is crucial in explaining sodium's chemical properties and reactivity. Atoms strive for stability by achieving a full outer shell, and sodium, with its single valence electron, can readily achieve this by losing that electron. Losing an electron transforms sodium into a positively charged ion, Na⁺, with a stable electron configuration identical to neon (Ne), a noble gas.
This propensity to lose an electron makes sodium highly reactive, particularly with elements that readily accept electrons, such as halogens (e.g., chlorine, bromine). The reaction between sodium and chlorine results in the formation of sodium chloride (NaCl), common table salt, through an ionic bond. In this bond, sodium loses its valence electron to chlorine, forming Na⁺ and Cl⁻ ions, which are electrostatically attracted to each other.
Sodium's Role in Biological Systems and Industrial Applications
Sodium's chemical properties, dictated by its single valence electron, are fundamental to its roles in various biological and industrial processes.
Biological Significance:
- Nerve impulse transmission: Sodium ions (Na⁺) play a vital role in the transmission of nerve impulses. Changes in sodium ion concentration across cell membranes generate electrical signals that allow for communication between nerve cells.
- Muscle contraction: Similar to nerve impulse transmission, sodium ions are crucial for muscle contraction. The movement of sodium ions across muscle cell membranes initiates the processes that lead to muscle fiber shortening and relaxation.
- Fluid balance: Sodium helps regulate the body's fluid balance. It contributes to the osmotic pressure of body fluids, which is crucial for maintaining the proper distribution of water within the body.
- Nutrient absorption: Sodium facilitates the absorption of certain nutrients in the digestive system.
Industrial Applications:
- Sodium lamps: Sodium vapor lamps, known for their bright yellow light, utilize sodium's properties for efficient light emission.
- Sodium hydroxide (NaOH) production: Sodium hydroxide, a strong base, is a crucial industrial chemical used in various applications, including paper production, soap manufacturing, and water treatment. It's produced through the electrolysis of sodium chloride.
- Sodium metal in alloys: Sodium metal is used in some alloys to modify their properties, such as improving their fluidity or strength.
Comparing Sodium with Other Alkali Metals
Sodium belongs to Group 1 of the periodic table, known as the alkali metals. All alkali metals share the characteristic of having one valence electron. This shared characteristic leads to similar chemical properties, including high reactivity and a tendency to form +1 ions. However, the reactivity and other properties vary slightly due to differences in atomic size and other factors:
| Alkali Metal | Atomic Number | Electronic Configuration | Valence Electrons | Reactivity |
|---|---|---|---|---|
| Lithium (Li) | 3 | 1s² 2s¹ | 1 | High |
| Sodium (Na) | 11 | 1s² 2s² 2p⁶ 3s¹ | 1 | High |
| Potassium (K) | 19 | 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ | 1 | High |
| Rubidium (Rb) | 37 | [Kr] 5s¹ | 1 | Very High |
| Cesium (Cs) | 55 | [Xe] 6s¹ | 1 | Very High |
| Francium (Fr) | 87 | [Rn] 7s¹ | 1 | Extremely High |
As we move down Group 1, the atomic size increases, and the valence electron is further from the nucleus, resulting in increased reactivity. Francium, being the largest alkali metal, is the most reactive.
Conclusion: Sodium's Single Valence Electron – A Key to its Properties
In summary, sodium possesses only one electron in its outer shell, making it a highly reactive element. This single valence electron's behavior dictates sodium's chemical properties, its tendency to form ionic bonds, and its crucial role in biological and industrial processes. Understanding the electronic configuration and the significance of valence electrons is fundamental to appreciating sodium's unique place in the chemical world and its importance to life and technology. The seemingly simple fact of sodium having one valence electron holds profound consequences in the vast realm of chemistry and beyond.
Latest Posts
Latest Posts
-
As You Like It Summary In 100 Words
Apr 02, 2025
-
Which Compound Has The Strongest Hydrogen Bonding Between Its Molecules
Apr 02, 2025
-
First I Threw Away The Outside And Cooked The Inside
Apr 02, 2025
-
Label The Parts Of The Nucleotide
Apr 02, 2025
-
Hyposecretion Of The Thyroid Gland In Adulthood
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Sodium Have In Its Outer Shell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
