Mass Of Hydrogen Atom In G
News Leon
Mar 30, 2025 · 5 min read
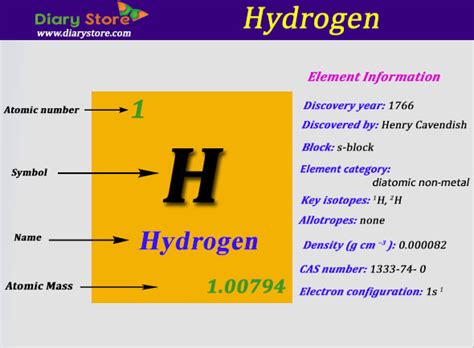
Table of Contents
The Mass of a Hydrogen Atom: A Deep Dive into Atomic Weight and its Implications
The seemingly simple question, "What is the mass of a hydrogen atom in grams?" opens a door to a fascinating exploration of atomic physics, chemistry, and the very foundations of our understanding of matter. While a quick search might offer a simple numerical answer, the true significance lies in understanding the nuances behind that number, its derivation, and its far-reaching implications across various scientific disciplines.
Understanding Atomic Mass Units (amu)
Before delving into the gram mass, it's crucial to understand the concept of atomic mass units (amu), also known as Daltons (Da). This unit is specifically designed to represent the mass of atoms and molecules. One amu is defined as one-twelfth the mass of a single, unbound neutral atom of carbon-12. This standardized unit provides a convenient scale for comparing the masses of different atoms and isotopes.
The mass of a hydrogen atom, specifically the most common isotope, protium (¹H), is approximately 1.00784 amu. This value isn't exactly 1 because it reflects the mass of the proton and a small contribution from the electron. The neutron, while present in heavier isotopes of hydrogen (deuterium and tritium), is absent in protium.
Converting amu to Grams: Avogadro's Number and the Mole
The conversion from amu to grams relies on a fundamental constant in chemistry: Avogadro's number. This number, approximately 6.022 x 10²³, represents the number of atoms or molecules in one mole of a substance. A mole is a unit of measurement that links the microscopic world of atoms and molecules to the macroscopic world of grams and kilograms that we experience daily.
The crucial relationship between amu and grams is:
1 amu ≈ 1.66054 x 10⁻²⁴ g
This conversion factor allows us to move seamlessly between the atomic mass unit scale and the gram scale we use in everyday life.
Calculating the Mass of a Hydrogen Atom in Grams
Now we can calculate the mass of a single hydrogen atom in grams:
Mass of ¹H in amu = 1.00784 amu
Mass of ¹H in grams = 1.00784 amu * (1.66054 x 10⁻²⁴ g/amu) ≈ 1.6737 x 10⁻²⁴ g
Therefore, the mass of a single hydrogen atom (protium) is approximately 1.6737 x 10⁻²⁴ grams.
Isotopes of Hydrogen and their Mass
It's important to remember that hydrogen has isotopes:
- Protium (¹H): One proton, zero neutrons. This is the most abundant isotope.
- Deuterium (²H or D): One proton, one neutron. Its mass is approximately 2.0141 amu, leading to a gram mass of roughly 3.3436 x 10⁻²⁴ g.
- Tritium (³H or T): One proton, two neutrons. Its mass is approximately 3.0160 amu, resulting in a gram mass of around 5.0082 x 10⁻²⁴ g.
The relative abundance of these isotopes affects the average atomic mass of hydrogen reported on the periodic table. The average atomic mass accounts for the weighted average of the masses of all naturally occurring isotopes.
The Significance of Atomic Mass in Chemistry and Physics
The accurate determination of atomic mass is fundamentally important across various scientific fields:
1. Stoichiometry and Chemical Reactions:
Accurate atomic masses are essential for stoichiometric calculations. These calculations allow us to determine the quantities of reactants and products involved in chemical reactions. Knowing the precise mass of each atom allows for precise predictions of reaction yields and compositions.
2. Nuclear Physics and Nuclear Reactions:
In nuclear physics, precise atomic mass measurements are crucial for understanding nuclear reactions, including nuclear fission and fusion. The mass defect (the difference between the mass of the nucleus and the sum of the masses of its constituent protons and neutrons) is related to the binding energy of the nucleus, a key concept in nuclear energy.
3. Mass Spectrometry:
Mass spectrometry is a powerful analytical technique that relies on determining the mass-to-charge ratio of ions. By accurately measuring the mass of ions, scientists can identify different molecules and isotopes. This technique has wide applications in various fields, including biochemistry, environmental science, and forensic science.
4. Astrophysics and Cosmology:
Precise atomic masses are also crucial in astrophysics and cosmology. Understanding the abundances of different elements in stars and galaxies relies heavily on accurate atomic mass data. These measurements provide insights into stellar nucleosynthesis and the evolution of the universe.
5. Material Science and Engineering:
The properties of materials are deeply intertwined with the mass and arrangement of atoms within their structures. Precise atomic mass data plays a role in understanding material behavior, such as strength, conductivity, and reactivity. This knowledge is vital in material science and engineering applications.
Beyond the Single Atom: Practical Applications and Considerations
While the mass of a single hydrogen atom is incredibly small, its implications are far-reaching when we consider larger quantities. The concept of the mole allows us to bridge the gap between the atomic scale and the macroscopic world. In everyday life and industrial applications, we work with moles of substances, not individual atoms.
Consider the following examples:
- Fuel cells: Hydrogen's low mass and high energy density make it an attractive fuel source for fuel cells. Understanding its atomic mass is crucial for designing efficient fuel cells.
- Fertilizers: Hydrogen is a vital component of ammonia, a key ingredient in many fertilizers. Precise knowledge of hydrogen's mass is essential for manufacturing fertilizers with the desired nitrogen content.
- Chemical synthesis: Many industrial chemical processes involve hydrogen as a reactant or product. Precise knowledge of its atomic mass is critical for optimizing yields and controlling reaction conditions.
Conclusion: A Tiny Atom, a Vast Impact
The seemingly insignificant mass of a single hydrogen atom, a mere 1.6737 x 10⁻²⁴ grams, has profound implications across a wide range of scientific and technological fields. Understanding its mass, its isotopic variations, and the relationships between amu, grams, and Avogadro's number, is fundamental to our understanding of chemistry, physics, and the world around us. This knowledge is not just an academic exercise; it forms the bedrock of numerous technological advancements and industrial processes that shape our modern world. The journey from a simple question about the mass of a hydrogen atom to the vast implications of that number highlights the power of fundamental scientific principles and their impact on our everyday lives.
Latest Posts
Latest Posts
-
The Chromosomes Are Aligned At The Spindle Equator During
Apr 01, 2025
-
Integers Are A Subset Of Rational Numbers True False
Apr 01, 2025
-
Draw The Structure Of Propanoic Acid
Apr 01, 2025
-
Is Water A Product Or Reactant
Apr 01, 2025
-
How Many Protons Are There In Any Chlorine Atom
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Mass Of Hydrogen Atom In G . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
