Is Water A Product Or Reactant
News Leon
Apr 01, 2025 · 6 min read
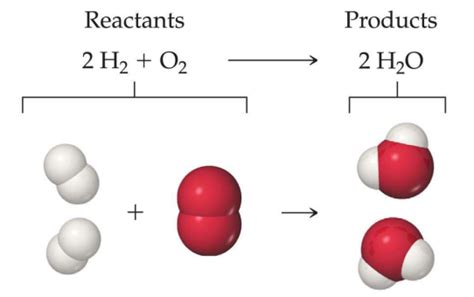
Table of Contents
Is Water a Product or Reactant? Understanding its Role in Chemical Reactions
Water, the elixir of life, is far more than just a simple molecule. Its ubiquitous presence in our world makes it easy to overlook its surprisingly complex and dynamic role in chemical reactions. Is water a product, a reactant, or both? The answer, as we'll explore in depth, is both, and understanding its multifaceted behavior is crucial to grasping fundamental chemical principles. This comprehensive exploration will delve into the diverse ways water participates in chemical processes, illustrating its importance in various contexts.
Water as a Reactant: Driving Chemical Change
In numerous chemical reactions, water acts as a reactant, meaning it actively participates in the transformation of other substances. This participation isn't passive; water molecules directly interact with reactants, influencing the reaction's pathway and outcome. Let's examine some key examples:
1. Hydrolysis Reactions: Breaking Bonds with Water
Hydrolysis reactions represent a significant category where water acts as a crucial reactant. The term itself, derived from the Greek words "hydro" (water) and "lysis" (to break), perfectly encapsulates the process. In hydrolysis, water molecules are added to a substance, causing its bonds to break, resulting in the formation of two or more new products. This is commonly seen in:
-
Ester hydrolysis: Esters, commonly found in fats and oils, undergo hydrolysis in the presence of water and an acid or base catalyst. The ester bond breaks, producing a carboxylic acid and an alcohol. This is a fundamental process in digestion, where enzymes catalyze the hydrolysis of fats.
-
Peptide bond hydrolysis: Proteins are long chains of amino acids linked by peptide bonds. Water's involvement in breaking these peptide bonds is essential for protein digestion. Enzymes facilitate this hydrolysis, releasing individual amino acids that the body can absorb.
-
Salt hydrolysis: When certain salts dissolve in water, they can undergo hydrolysis, leading to the formation of acidic or basic solutions. This happens when the cation or anion of the salt reacts with water, altering the solution's pH. For example, the hydrolysis of sodium acetate results in a slightly basic solution.
2. Hydration Reactions: Adding Water Molecules
Hydration reactions involve the addition of water molecules to a substance, often leading to the formation of hydrates. This process often alters the physical properties of the substance, such as its solubility and crystal structure. Anhydrous copper(II) sulfate, for instance, is a white powder. When hydrated, it absorbs water molecules to form copper(II) sulfate pentahydrate, a vibrant blue crystal. This change in color serves as a visual indicator of the hydration reaction.
3. Acid-Base Reactions: Water's Amphoteric Nature
Water's unique amphoteric nature—its ability to act as both an acid and a base—plays a critical role in acid-base reactions. It can donate a proton (H⁺) acting as an acid or accept a proton acting as a base. This dual capability contributes significantly to the self-ionization of water, an essential concept in understanding pH and solution acidity.
-
Autoionization of water: In pure water, a small fraction of molecules undergo autoionization, where one water molecule acts as an acid, donating a proton to another water molecule acting as a base. This produces hydronium ions (H₃O⁺) and hydroxide ions (OH⁻), establishing an equilibrium that defines the pH of pure water (7).
-
Neutralization reactions: Acid-base neutralization reactions often involve water as a product. When an acid and a base react, they neutralize each other, forming water and a salt. This is a classic example of water's role in achieving chemical equilibrium.
Water as a Product: The Outcome of Chemical Reactions
Water's role isn't confined to being a reactant; it frequently appears as a product in various chemical processes. The formation of water often signifies the completion of a reaction or a crucial step in a larger reaction sequence.
1. Combustion Reactions: Water as a Byproduct of Oxidation
Combustion reactions, which involve the rapid oxidation of a fuel, often produce water as a byproduct. The burning of hydrocarbons, such as methane (CH₄) or propane (C₃H₈), in the presence of oxygen yields carbon dioxide (CO₂) and water (H₂O). This is a highly exothermic process, releasing a significant amount of energy. This process is fundamental in energy generation and various industrial applications.
2. Acid-Base Neutralization: Water as a Key Product
As mentioned earlier, the neutralization reaction between an acid and a base always produces water as a product, along with a salt. This reaction is fundamental in chemistry, representing a crucial method for preparing salts and controlling the pH of solutions. The quantitative relationship between acid, base, and water in neutralization reactions is described by stoichiometry.
3. Dehydration Reactions: Water Removal as a Product
Dehydration reactions, in contrast to hydrolysis, involve the removal of water molecules from a reactant. This process typically creates a double bond or a ring structure within the molecule. For example, the dehydration of alcohols often yields alkenes, while the dehydration of sugars can lead to the formation of cyclic structures. The water molecule removed appears as a separate product in these reactions.
4. Redox Reactions: Water Formation Through Oxidation and Reduction
Water can also be formed as a product in redox reactions, which involve electron transfer between chemical species. In many redox reactions involving oxygen or hydrogen, water molecules are created as a result of the reduction of oxygen or the oxidation of hydrogen. For example, in the electrolysis of water, water is split into its constituent elements—hydrogen and oxygen—and the reverse process can be viewed as a redox reaction forming water.
The Importance of Water's Role: Beyond Simple Reactions
Understanding water's dual role as both a reactant and a product extends beyond the basic chemical equations. It has profound implications across various disciplines:
-
Biological systems: Water is the solvent of life. Its ability to participate in hydrolysis and hydration reactions is crucial for biochemical processes, including digestion, enzyme function, and cellular transport.
-
Environmental chemistry: Water's role in reactions influences its quality and impact on the environment. Acid rain, for instance, is caused by the reaction of atmospheric pollutants with water, forming acidic solutions that damage ecosystems.
-
Industrial processes: Many industrial chemical processes rely on water as a reactant, solvent, or cooling agent. Understanding water's behavior in these processes is essential for optimizing efficiency and minimizing environmental impact.
-
Geochemical processes: Water plays a crucial role in weathering, erosion, and the formation of minerals. Its interaction with rocks and minerals through dissolution and precipitation reactions shapes the Earth's landscape.
Conclusion: Water's Dynamic Participation in the Chemical World
Water, far from being an inert substance, actively participates in a vast array of chemical reactions, acting as both a reactant and a product. Its unique properties, particularly its amphoteric nature and high polarity, make it a crucial component in countless natural and industrial processes. A deep understanding of water's diverse roles is essential for comprehending fundamental chemical principles and tackling complex challenges across multiple scientific disciplines. Further research into water's behavior under varying conditions continues to unlock new insights into its profound influence on our world. From the microscopic level of cellular processes to the macroscopic scale of geological formations, water's ubiquitous presence and dynamic chemical nature are undeniable and continue to be a subject of fascination and exploration.
Latest Posts
Latest Posts
-
Value Of Time Essay 1000 Words
Apr 02, 2025
-
Why Is Ice Melting Not A Chemical Reaction
Apr 02, 2025
-
Which Of The Following Is A Property Of A Solid
Apr 02, 2025
-
The Law Of Diminishing Marginal Utility States That The
Apr 02, 2025
-
What Is An Opening Balance Sheet
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Product Or Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
