How Many Protons Are There In Any Chlorine Atom
News Leon
Apr 01, 2025 · 5 min read
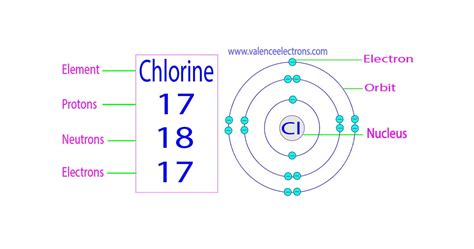
Table of Contents
How Many Protons Are There in Any Chlorine Atom? A Deep Dive into Atomic Structure
Understanding the fundamental building blocks of matter is crucial in chemistry and physics. One key aspect of this understanding involves the atomic structure of elements, specifically the number of protons, neutrons, and electrons within an atom. This article will delve into the specific case of chlorine, answering the question: how many protons are there in any chlorine atom? We'll explore the concepts of atomic number, isotopes, and the implications of proton count on chlorine's properties.
Understanding Atomic Number and Chlorine's Place on the Periodic Table
The number of protons in an atom's nucleus defines its atomic number and ultimately its identity as a specific element. This number is unique to each element and is what distinguishes chlorine from all other elements. You'll find this number listed on the periodic table, a cornerstone of chemistry. The periodic table arranges elements based on their atomic number and recurring chemical properties.
Chlorine (Cl), located in Group 17 (also known as the halogens) and Period 3 of the periodic table, boasts an atomic number of 17. This definitively means that every chlorine atom contains 17 protons. This is a fundamental truth; without 17 protons, an atom simply cannot be classified as chlorine.
The Significance of Protons in Defining an Element
Protons, along with neutrons, constitute the atom's nucleus, representing the bulk of its mass. However, it's the number of protons, not neutrons or electrons, that determines the element's identity. The number of protons dictates the positive charge of the nucleus, which in turn determines the number of electrons required to maintain electrical neutrality in a neutral atom. This electron configuration directly influences the element's chemical behavior and reactivity.
Isotopes: Variations in Neutron Count, Constant Proton Count
While the number of protons remains constant for a given element, the number of neutrons can vary. These variations create different isotopes of the same element. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This means they have the same atomic number but different mass numbers (the sum of protons and neutrons).
Chlorine has two naturally occurring, stable isotopes:
- Chlorine-35 (³⁵Cl): This isotope accounts for approximately 76% of naturally occurring chlorine. It contains 17 protons and 18 neutrons (17 + 18 = 35).
- Chlorine-37 (³⁷Cl): This isotope makes up about 24% of naturally occurring chlorine. It also contains 17 protons but has 20 neutrons (17 + 20 = 37).
Crucially, both isotopes of chlorine possess 17 protons. The difference lies solely in their neutron count, which affects their mass but not their fundamental chemical properties. While the mass difference influences certain physical properties like diffusion rates, the chemical reactivity of both isotopes is essentially identical because the number of electrons and their arrangement remain the same.
The Role of Electrons and Chemical Reactivity
While protons define the element, electrons are responsible for its chemical behavior. A neutral chlorine atom possesses 17 electrons, balancing the positive charge of the 17 protons. However, chlorine is highly reactive because it readily gains one electron to achieve a stable octet (eight electrons) in its outermost electron shell. This tendency to gain an electron is what makes chlorine such a strong oxidizing agent.
This electron gain leads to the formation of a chloride ion (Cl⁻), which has a stable electron configuration identical to that of argon (Ar), a noble gas. The acquisition of that extra electron isn't changing the number of protons – only the electron count and thus the charge of the atom.
Chlorine's Chemical Properties and Applications
Chlorine's reactivity makes it crucial in various applications:
- Water Treatment: Chlorine is widely used to disinfect drinking water and swimming pools, killing harmful bacteria and viruses.
- Industrial Processes: It's a vital component in the production of plastics (like PVC), solvents, and other chemicals.
- Bleach Production: Chlorine is used in the manufacturing of bleach, a powerful oxidizing agent.
These applications underscore the importance of understanding chlorine's atomic structure. The 17 protons dictate its chemical properties, leading to its versatile applications.
Beyond Chlorine: The Importance of Atomic Number Across the Periodic Table
The principle established with chlorine—that the atomic number equals the number of protons—holds true for all elements. This fundamental concept underpins our understanding of the periodic table and the predictable behavior of elements. Each element's unique atomic number and resulting electron configuration dictate its physical and chemical properties, making it possible to predict how elements will interact and form compounds.
The periodic table itself is organized around atomic number, allowing scientists to readily compare and contrast the characteristics of different elements. Elements in the same group share similar electron configurations in their outermost shell, leading to similar chemical reactivity. Elements in the same period have the same number of electron shells.
Exploring Other Elements and Their Proton Counts
Let's briefly consider a few examples to illustrate the universality of the proton-atomic number relationship:
- Hydrogen (H): Atomic number 1, meaning 1 proton.
- Oxygen (O): Atomic number 8, meaning 8 protons.
- Iron (Fe): Atomic number 26, meaning 26 protons.
- Gold (Au): Atomic number 79, meaning 79 protons.
Conclusion: The Invariant Nature of Protons in Defining Chlorine
In conclusion, the number of protons in a chlorine atom is consistently and unequivocally 17. This unchanging number is what fundamentally defines chlorine as a distinct element, separate from all others on the periodic table. While the number of neutrons may vary (creating isotopes), and the number of electrons can change (resulting in ions), the proton count remains constant and is the defining characteristic of every chlorine atom. This unwavering presence of 17 protons is crucial in understanding chlorine's chemical behavior, reactivity, and its many essential applications in various fields. The principle of a fixed proton count for each element applies universally across the periodic table, forming the basis of modern chemistry and our understanding of matter itself.
Latest Posts
Latest Posts
-
Why Is Ice Melting Not A Chemical Reaction
Apr 02, 2025
-
Which Of The Following Is A Property Of A Solid
Apr 02, 2025
-
The Law Of Diminishing Marginal Utility States That The
Apr 02, 2025
-
What Is An Opening Balance Sheet
Apr 02, 2025
-
As You Like It Summary In 100 Words
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Are There In Any Chlorine Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
