One Atomic Mass Unit Is Equal To
News Leon
Mar 25, 2025 · 6 min read
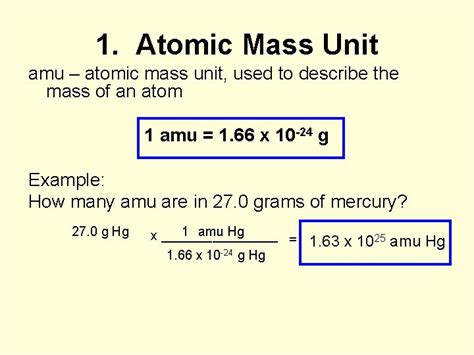
Table of Contents
One Atomic Mass Unit is Equal To: A Deep Dive into Atomic Mass
The seemingly simple question, "One atomic mass unit is equal to what?", opens a door to a fascinating world of atomic physics, chemistry, and the very building blocks of matter. Understanding the atomic mass unit (amu) is crucial for anyone studying science at a higher level, from high school chemistry to advanced quantum mechanics. This comprehensive guide will explore the definition, history, applications, and significance of the amu, providing a detailed explanation that’s both informative and accessible.
Defining the Atomic Mass Unit (amu)
The atomic mass unit (amu), also known as a dalton (Da), is a standard unit of mass used to express atomic and molecular weights. It's defined as one-twelfth the mass of a single unbound neutral atom of carbon-12 (¹²C) in its nuclear and electronic ground state. This means:
1 amu = 1/12 the mass of a ¹²C atom
This definition is crucial because it provides a consistent and universally accepted standard for measuring the mass of atoms and molecules. Prior to this standardized definition, scientists relied on various relative scales, leading to inconsistencies and confusion. The adoption of the ¹²C standard in 1961 brought much-needed uniformity to the field.
Why Carbon-12?
The choice of carbon-12 as the standard is not arbitrary. Several factors contributed to this decision:
- Abundance: Carbon-12 is relatively abundant in nature, making it readily available for measurements.
- Stability: It's a stable isotope, ensuring consistent mass measurements.
- Measurability: Its mass is easily measurable with high precision using mass spectrometry.
Using carbon-12 provides a solid foundation for calculating the atomic masses of all other elements. Because the mass of a carbon-12 atom is known with high accuracy, the mass of other atoms can be determined relative to it.
The Numerical Value of One Atomic Mass Unit
While the definition clarifies what an amu is relative to carbon-12, the question remains: what is its numerical value in more familiar units like grams or kilograms?
1 amu ≈ 1.66053906660 × 10⁻²⁷ kg
1 amu ≈ 1.66053906660 × 10⁻²⁴ g
This incredibly small number highlights the minuscule scale at which atoms and molecules operate. It’s important to note that this is an approximate value; the exact numerical value depends on the precision of the measurement techniques used.
Calculating Atomic Mass with amu
The atomic mass of an element is the weighted average of the masses of its isotopes, taking into account their relative abundances. This means that the atomic mass reflects the average mass of an atom of that element as it exists in nature. The atomic mass is expressed in amu.
For instance, chlorine has two main isotopes: ³⁵Cl (75.77% abundance) and ³⁷Cl (24.23% abundance). To calculate the atomic mass of chlorine:
(0.7577 * 34.96885 amu) + (0.2423 * 36.96590 amu) ≈ 35.45 amu
This calculated atomic mass of approximately 35.45 amu is the value you’ll find listed on the periodic table for chlorine. This illustrates how amu is used practically in calculating the average mass of atoms found naturally.
Applications of the Atomic Mass Unit
The atomic mass unit is fundamental to numerous fields, including:
Chemistry
- Stoichiometry: amu is essential for calculating the molar mass of compounds and performing stoichiometric calculations, crucial for balancing chemical equations and determining reaction yields.
- Molecular Weight Determination: The amu is the basic unit for expressing molecular weight, which is the sum of the atomic masses of all the atoms in a molecule.
- Spectroscopy: Mass spectrometry relies heavily on the amu to identify and quantify different isotopes and molecules based on their mass-to-charge ratios.
Physics
- Nuclear Physics: amu is vital for understanding nuclear reactions, decay processes, and the binding energies of atomic nuclei.
- Particle Physics: The amu is used in calculations involving subatomic particles and their masses.
Biochemistry & Molecular Biology
- Protein Mass Determination: Determining the mass of proteins and other biomolecules using techniques like mass spectrometry relies on the amu.
- DNA Sequencing: Understanding the mass of DNA fragments assists in DNA sequencing and analysis.
Material Science
- Material Characterization: Determining the atomic composition and mass of materials is fundamental to understanding their properties.
The Significance of the Atomic Mass Unit
The amu's significance extends beyond its practical applications. Its consistent and universally accepted definition has revolutionized scientific communication and collaboration. The ability to express atomic and molecular masses using a standardized unit eliminates ambiguity and allows for precise comparisons of data across different laboratories and research groups. This standardized unit facilitates more accurate and reliable scientific research across the globe.
Furthermore, the amu provides a crucial link between the microscopic world of atoms and molecules and the macroscopic world of everyday measurements. It bridges the gap between the incredibly small masses of individual atoms and the larger-scale quantities that we typically work with in experiments and industrial processes.
Beyond the Basics: Isotopic Abundance and Weighted Average Mass
We've touched on the concept of isotopic abundance and its role in calculating the atomic mass. Let's delve deeper.
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number leads to variations in the atom's mass. For example, carbon has two stable isotopes: ¹²C and ¹³C. ¹²C has six protons and six neutrons, while ¹³C has six protons and seven neutrons.
The atomic mass listed in the periodic table is a weighted average of the masses of all the naturally occurring isotopes of an element. This weight is determined by the relative abundance of each isotope. The more abundant an isotope is, the greater its contribution to the overall atomic mass.
To calculate the weighted average atomic mass, you multiply the mass of each isotope (in amu) by its fractional abundance and then sum the products:
Average Atomic Mass = Σ (mass of isotope * fractional abundance)
For example, consider chlorine again:
- ³⁵Cl: mass = 34.96885 amu, abundance = 0.7577
- ³⁷Cl: mass = 36.96590 amu, abundance = 0.2423
Average Atomic Mass = (34.96885 amu * 0.7577) + (36.96590 amu * 0.2423) ≈ 35.45 amu
This weighted average mass is the value reported in the periodic table, reflecting the average mass of a chlorine atom as it occurs in nature.
Connecting amu to Moles and Avogadro's Number
The atomic mass unit is intimately connected to the mole, a crucial concept in chemistry. One mole of a substance contains Avogadro's number (approximately 6.022 x 10²³) of entities (atoms, molecules, ions, etc.). The molar mass of a substance is the mass of one mole of that substance, expressed in grams.
Importantly, the numerical value of the molar mass of a substance is numerically equal to its atomic or molecular weight expressed in amu. For example, the atomic weight of carbon is approximately 12 amu. This means that one mole of carbon has a mass of approximately 12 grams. This equivalence is a direct consequence of the definition of the amu and Avogadro's number. It's a cornerstone of stoichiometric calculations and is essential for relating the microscopic world to the macroscopic world in chemistry.
Conclusion
One atomic mass unit is not simply a number; it's a fundamental unit underpinning our understanding of matter at the atomic and molecular level. From its carefully chosen definition based on carbon-12 to its numerous applications in chemistry, physics, and biology, the amu plays a pivotal role in modern science. Its importance extends beyond calculations; it serves as a unifying standard, fostering collaboration and precision in scientific research across various disciplines. Understanding the amu, therefore, is crucial for anyone seeking a deeper understanding of the world at its most fundamental level.
Latest Posts
Latest Posts
-
Every Computer Has An Operating System
Mar 25, 2025
-
Which Of The Following Is A Browser
Mar 25, 2025
-
What Is The Conjugate Base Of Nh4
Mar 25, 2025
-
How Many Years Did Rip Van Winkle Sleep
Mar 25, 2025
-
Number Of Valence Electrons In Calcium
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about One Atomic Mass Unit Is Equal To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
