Acetic Acid Reacts With Sodium Hydroxide
News Leon
Mar 25, 2025 · 5 min read
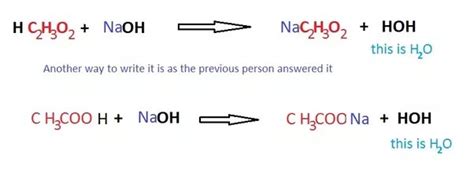
Table of Contents
Acetic Acid Reacts with Sodium Hydroxide: A Deep Dive into Acid-Base Chemistry
Acetic acid, the main component of vinegar, and sodium hydroxide, a common strong base, readily react in a classic acid-base neutralization reaction. This seemingly simple reaction offers a rich tapestry of chemical principles, applications, and practical implications. Understanding this reaction provides a foundational understanding of acid-base chemistry, stoichiometry, and titration techniques. This comprehensive article delves into the intricacies of this reaction, exploring its mechanism, applications, and related concepts.
The Reaction: A Neutralization Process
The reaction between acetic acid (CH₃COOH) and sodium hydroxide (NaOH) is a classic example of an acid-base neutralization reaction. Acetic acid, a weak acid, donates a proton (H⁺) to the strong base, sodium hydroxide, which accepts the proton. This results in the formation of water (H₂O) and sodium acetate (CH₃COONa), a salt.
The balanced chemical equation is:
CH₃COOH(aq) + NaOH(aq) → CH₃COONa(aq) + H₂O(l)
Understanding the Reactants
-
Acetic Acid (CH₃COOH): A weak organic acid, meaning it only partially dissociates in water. This partial dissociation is key to understanding its behavior in the reaction with sodium hydroxide. The equilibrium lies significantly to the left, meaning a substantial amount of undissociated acetic acid remains in solution.
-
Sodium Hydroxide (NaOH): A strong inorganic base, meaning it completely dissociates in water into sodium (Na⁺) and hydroxide (OH⁻) ions. The abundance of hydroxide ions drives the reaction with acetic acid.
Understanding the Products
-
Sodium Acetate (CH₃COONa): A salt formed from the neutralization reaction. It's a relatively neutral compound in solution, though its ionic nature can slightly affect the pH. The acetate ion (CH₃COO⁻) is the conjugate base of acetic acid.
-
Water (H₂O): The formation of water is a hallmark of acid-base neutralization reactions. The proton from the acetic acid combines with the hydroxide ion from sodium hydroxide to form water.
The Mechanism: A Step-by-Step Breakdown
The reaction proceeds through a simple proton transfer mechanism. The hydroxide ion (OH⁻) from the completely dissociated sodium hydroxide acts as a strong nucleophile, attacking the slightly positive hydrogen atom of the carboxylic acid group in acetic acid.
-
Proton Transfer: The hydroxide ion abstracts a proton from the acetic acid molecule. This creates a water molecule and an acetate ion.
-
Ion Formation: The acetate ion (CH₃COO⁻) carries a negative charge and the sodium ion (Na⁺) from the sodium hydroxide remains in solution.
Stoichiometry: The Quantitative Aspect
Stoichiometry plays a crucial role in understanding the quantitative relationships between reactants and products. The balanced chemical equation shows a 1:1 molar ratio between acetic acid and sodium hydroxide. This means that one mole of acetic acid reacts completely with one mole of sodium hydroxide.
This 1:1 stoichiometry is vital in titration experiments, where the concentration of an unknown solution (either acetic acid or sodium hydroxide) can be determined by reacting it with a solution of known concentration.
Titration: A Practical Application
Titration is a common laboratory technique used to determine the concentration of a solution. In the case of acetic acid and sodium hydroxide, a titration involves slowly adding a solution of known concentration (the titrant, often sodium hydroxide) to a solution of unknown concentration (the analyte, often acetic acid) until the reaction is complete.
The endpoint of the titration, signifying complete neutralization, is typically indicated by a change in color using a suitable indicator, such as phenolphthalein. Phenolphthalein is colorless in acidic solutions and turns pink in basic solutions. The point at which the solution turns from colorless to a faint pink indicates that all the acetic acid has been neutralized.
By knowing the volume and concentration of the sodium hydroxide solution used, and the volume of the acetic acid solution, one can calculate the concentration of the acetic acid using the stoichiometry of the reaction.
pH Changes During the Reaction
Monitoring the pH change during the titration provides valuable insights into the reaction. Initially, the pH of the acetic acid solution is acidic (below 7). As sodium hydroxide is added, the pH gradually increases.
The point at which the pH changes most rapidly is the equivalence point, indicating complete neutralization. For the reaction between acetic acid and sodium hydroxide, the equivalence point is at a pH slightly above 7 due to the acetate ion's weak basicity. Beyond the equivalence point, further addition of sodium hydroxide results in a sharp increase in pH.
Applications of the Reaction
The reaction between acetic acid and sodium hydroxide finds numerous applications across various fields:
-
Buffer Solutions: The reaction can be used to prepare buffer solutions. A buffer solution resists changes in pH upon addition of small amounts of acid or base. A mixture of acetic acid and sodium acetate acts as a buffer solution, maintaining a relatively stable pH around the pKa of acetic acid (4.76).
-
Food Industry: The reaction is relevant in food processing and preservation. Acetic acid is often neutralized using sodium hydroxide to adjust the pH of food products.
-
Chemical Synthesis: Sodium acetate, a product of the reaction, is used as a reactant in many organic synthesis reactions.
-
Wastewater Treatment: Neutralization reactions, including this one, are employed in wastewater treatment to adjust the pH of wastewater to prevent environmental damage.
Safety Precautions
While this reaction is relatively safe, certain safety precautions should always be followed:
-
Eye Protection: Always wear safety goggles to protect your eyes from splashes of chemicals.
-
Gloves: Wear gloves to protect your hands from contact with the chemicals.
-
Proper Ventilation: Perform the reaction in a well-ventilated area to avoid inhaling fumes.
-
Disposal: Dispose of the waste properly according to local regulations.
Conclusion: A Foundational Reaction
The reaction between acetic acid and sodium hydroxide serves as an excellent example of a fundamental acid-base neutralization reaction. Its simplicity belies its significance, providing valuable insights into stoichiometry, titration techniques, pH changes, and the properties of weak acids and strong bases. Understanding this reaction forms a solid foundation for exploring more complex chemical systems and applications. Its widespread use in various fields highlights its practical importance and enduring relevance in chemistry. The reaction's simplicity makes it an ideal starting point for learning about acid-base chemistry, and its applications showcase its practical relevance in everyday life and industry. Further exploration of this reaction can lead to a deeper understanding of equilibrium, kinetics, and other key concepts in chemistry.
Latest Posts
Latest Posts
-
The Smallest Unit That Can Evolve Is
Mar 25, 2025
-
Dna Is Composed Of Repeating Structural Units Called
Mar 25, 2025
-
Which Substance Loses Electrons In A Chemical Reaction
Mar 25, 2025
-
1 Mg Is How Many Units
Mar 25, 2025
-
Uaa Uga And Uag Are All Codons
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Acetic Acid Reacts With Sodium Hydroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
