Molarity Of Water In Pure Water
News Leon
Mar 20, 2025 · 5 min read
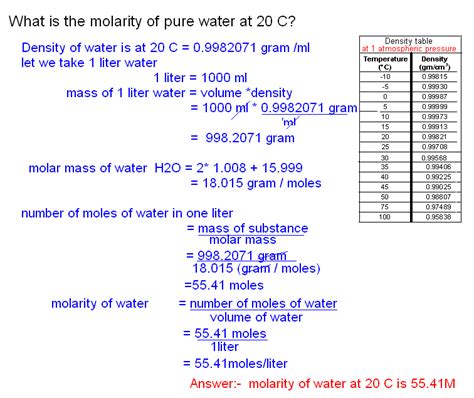
Table of Contents
Molarity of Water in Pure Water: A Deep Dive
The concept of molarity, a measure of concentration representing moles of solute per liter of solution, is fundamental in chemistry. While often applied to solutions of dissolved substances, it's equally valid—though perhaps less intuitively considered—to calculate the molarity of water itself in pure water. Understanding this seemingly simple calculation reveals deeper insights into the properties of water and its behavior in various contexts. This article delves into the calculation, explores its implications, and addresses common misconceptions.
Defining Molarity and its Application to Pure Water
Molarity (M) is defined as the number of moles of solute present per liter of solution. The formula is:
Molarity (M) = Moles of solute / Liters of solution
In a solution, the solute is the substance dissolved, and the solvent is the substance doing the dissolving. However, in pure water, water acts as both the solute and the solvent. This apparent paradox simplifies the calculation. We consider water as the solute because we're interested in its concentration as a component of the system.
To calculate the molarity of water, we need two pieces of information:
-
The molar mass of water: Water (H₂O) has a molar mass of approximately 18.015 g/mol (1.008 g/mol for hydrogen x 2 + 15.999 g/mol for oxygen).
-
The density of water: The density of water is approximately 1 g/mL or 1000 g/L at standard temperature and pressure (STP, 0°C and 1 atm). Slight variations exist at different temperatures and pressures, but for our purposes, this approximation suffices.
Calculating the Molarity of Water
Let's break down the calculation step-by-step:
-
Determine the mass of water in 1 liter: Since the density of water is 1 g/mL or 1000 g/L, 1 liter of pure water has a mass of 1000 grams.
-
Convert grams to moles: Using the molar mass of water (18.015 g/mol), we can convert the mass of water to moles:
(1000 g) / (18.015 g/mol) ≈ 55.51 moles
-
Calculate molarity: Now we can apply the molarity formula:
Molarity = Moles of water / Liters of water = 55.51 moles / 1 L = 55.51 M
Therefore, the molarity of water in pure water is approximately 55.51 M. This is a remarkably high concentration, highlighting the immense number of water molecules present in even a small volume of water.
Understanding the Implications of the High Molarity
The high molarity of water (55.51 M) has several important implications:
-
Concentration in reactions: In chemical reactions involving water as a reactant or solvent, the high concentration of water significantly influences reaction kinetics and equilibrium. The water concentration is often considered to be constant and incorporated into the equilibrium constant.
-
Solvent properties: The high concentration of water molecules directly impacts its exceptional solvent properties. The polar nature of water molecules and their ability to form hydrogen bonds contribute to its ability to dissolve a wide range of substances. The sheer number of water molecules enhances this ability.
-
Understanding solution stoichiometry: When calculating the concentration of other substances in aqueous solutions, the molarity of water provides a reference point for understanding the relative proportions of different components in the system.
-
Applications in various fields: The molarity of water plays a role in various fields, including environmental science, biochemistry, and materials science. For example, understanding water's concentration is crucial in determining the solubility of pollutants in water bodies and analyzing biological processes where water is the primary solvent.
Addressing Common Misconceptions
Several misconceptions surround the molarity of water in pure water. Let's clarify some of them:
-
"Pure water has no molarity": This is incorrect. Molarity is a measure of concentration, and even pure water has a defined concentration of water molecules.
-
"The molarity of water is 1 M": This is a common mistake. It arises from confusing the concept of molarity with the density of water (approximately 1 g/mL). Molarity involves moles, not mass.
-
"Molarity is only applicable to solutions": This is also incorrect. While molarity is frequently used for solutions, it's fundamentally a measure of concentration that can be applied to any substance, including pure substances like water.
Practical Applications and Further Exploration
The concept of water's molarity extends beyond theoretical calculations. It finds practical application in several areas:
-
Chemical Engineering: In processes involving water as a solvent or reactant, precise understanding of water's concentration is essential for process optimization and yield predictions.
-
Environmental Chemistry: Determining the concentration of pollutants in water bodies requires considering the high molarity of water as a background concentration.
-
Biochemistry and Physiology: In biological systems, water's high molarity is a key determinant of various biochemical reactions and processes.
-
Analytical Chemistry: In titrations and other analytical procedures involving aqueous solutions, the high concentration of water is often implicitly considered in the calculations.
Beyond Molarity: Other Concentration Units
While molarity is a useful measure of concentration, several other concentration units exist, each with its own advantages and disadvantages. These include:
-
Molality: Defined as moles of solute per kilogram of solvent. Unlike molarity, molality is not temperature-dependent.
-
Normality: Defined as the number of equivalents of solute per liter of solution. This is particularly useful for acid-base and redox reactions.
-
Mole fraction: The ratio of the number of moles of one component to the total number of moles of all components in the solution.
-
Mass percentage: The mass of the solute divided by the total mass of the solution, multiplied by 100%.
The choice of the most appropriate concentration unit depends on the specific application and the nature of the solution or substance being considered.
Conclusion: A Fundamental Concept with Broad Implications
The seemingly simple calculation of the molarity of water in pure water—approximately 55.51 M—reveals a fundamental aspect of water's chemical nature and its significant role in numerous chemical and biological processes. Understanding this high molarity is essential for interpreting chemical reactions, analyzing solutions, and appreciating water's unique properties as a solvent and reactant. This concept serves as a cornerstone for many advanced chemical and scientific endeavors. By grasping the molarity of water, we gain a deeper appreciation for the fundamental principles governing the behavior of matter at the molecular level.
Latest Posts
Latest Posts
-
A Water Intake At A Pump Storage Reservoir
Mar 21, 2025
-
In The Figure A Constant Force Fa Of Magnitude
Mar 21, 2025
-
The Inspiratory And Expiratory Centers Are Located In The
Mar 21, 2025
-
Cells Are The Basic Structural Units Of Living Organisms Explain
Mar 21, 2025
-
What Are The 3 Parts Of An Atp Molecule
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Molarity Of Water In Pure Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
