Like Electric Charges Repel Each Other. True Or False
News Leon
Mar 17, 2025 · 6 min read
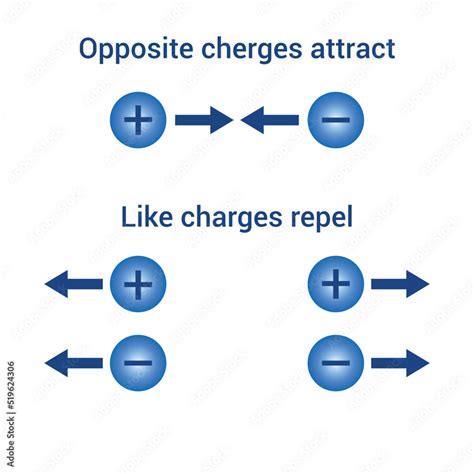
Table of Contents
Like Electric Charges Repel Each Other: True or False? Exploring Coulomb's Law and Electrostatics
The statement "like electric charges repel each other" is true. This fundamental principle of electrostatics governs the interactions between charged particles and forms the bedrock of many technologies we rely on daily. Understanding this principle requires delving into the nature of electric charge and Coulomb's Law, which mathematically describes the force between these charges. This article will explore this concept in depth, explaining the underlying physics, providing real-world examples, and addressing potential misconceptions.
Understanding Electric Charge
Before exploring the repulsion of like charges, it's crucial to understand the concept of electric charge itself. Electric charge is a fundamental property of matter, much like mass. It exists in two forms: positive and negative. These charges are not arbitrary labels; they represent a fundamental distinction in the behavior of subatomic particles.
- Protons, found in the nucleus of an atom, carry a positive charge.
- Electrons, orbiting the nucleus, carry a negative charge.
- Neutrons, also in the nucleus, are electrically neutral, carrying no charge.
The net charge of an object is determined by the balance (or imbalance) between the number of protons and electrons it contains. An object with an equal number of protons and electrons is electrically neutral. However, if an object loses electrons, it acquires a net positive charge (becoming a positive ion). Conversely, if an object gains electrons, it acquires a net negative charge (becoming a negative ion). This transfer of electrons is the basis of many electrical phenomena.
The Significance of Charge Quantization
It's important to note that electric charge is quantized. This means that charge exists in discrete units, rather than continuously variable amounts. The fundamental unit of charge is the elementary charge, denoted by 'e', and is the magnitude of the charge carried by a single proton or electron. This quantization is a direct consequence of the quantum nature of matter.
Coulomb's Law: The Mathematical Description of Charge Interaction
Coulomb's Law provides a precise mathematical description of the electrostatic force between two point charges. It states that the force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The formula is expressed as:
F = k * |q1 * q2| / r²
Where:
- F represents the electrostatic force between the charges.
- k is Coulomb's constant (approximately 8.98755 × 10⁹ N⋅m²/C²).
- q1 and q2 are the magnitudes of the two charges.
- r is the distance between the centers of the two charges.
The crucial aspect of this law, relevant to our topic, is the sign of the force. The formula uses the absolute values of the charges; the sign of the force is determined by the signs of the charges themselves.
- Like charges repel: If q1 and q2 have the same sign (both positive or both negative), the force F is positive, indicating a repulsive force. This confirms the truth of the statement: like charges repel.
- Opposite charges attract: If q1 and q2 have opposite signs (one positive and one negative), the force F is negative, indicating an attractive force.
Real-World Examples of Like Charge Repulsion
The repulsion of like charges is not just a theoretical concept; it's a phenomenon readily observable in the real world. Here are a few examples:
1. Static Cling:
Have you ever noticed how clothes sometimes cling together after being dried in a machine? This is often due to static electricity. The friction during the drying process can transfer electrons from one garment to another, leaving some positively charged and others negatively charged. Like charges on different pieces of clothing will repel each other, leading to that annoying clinging effect.
2. Van de Graaff Generator:
A Van de Graaff generator is a device that builds up a large static charge on a metal sphere. If you touch the sphere, your hair stands on end. This happens because the charge transferred to you from the generator repels like charges in your hair, causing the strands to push away from each other.
3. Electrostatic Precipitation:
This technology utilizes the principle of charge repulsion to remove pollutants from industrial exhaust gases. The pollutants are given a charge, and then they are attracted to oppositely charged plates, separating them from the clean gas stream. Repulsion between like-charged pollutants further contributes to efficient separation.
4. Lightning:
While lightning involves a complex interplay of charges, the repulsion of like charges plays a crucial role. The build-up of excess charge in clouds leads to a significant potential difference between different regions within the cloud or between the cloud and the ground. The repulsion of like charges within the cloud contributes to the creation of powerful electrical discharges that we observe as lightning.
Addressing Misconceptions
Several misconceptions surround electrostatic interactions. Let's address a few:
1. Charge Size vs. Repulsion Strength:
The magnitude of the charge directly influences the strength of the repulsion. Larger charges will repel each other more strongly than smaller charges at the same distance.
2. Distance and Repulsion:
The distance between the charges significantly impacts the repulsive force. As the distance increases, the repulsive force decreases rapidly (inverse square law). This explains why static cling is more noticeable when clothes are close together.
Beyond Point Charges: Real-World Objects
Coulomb's Law strictly applies to point charges – idealized charges with negligible size. However, the principles remain relevant for larger objects. The force between extended charged objects can be calculated by considering the interaction of individual charge elements within the objects and integrating over their entire volume. This often leads to complex calculations but the underlying principle of like charges repelling remains constant.
Conclusion: The Importance of Understanding Like-Charge Repulsion
The principle that like electric charges repel each other is a fundamental pillar of electrostatics and has far-reaching implications in various fields, from everyday phenomena like static cling to advanced technologies like electrostatic precipitation. Understanding this principle is key to comprehending a wide range of electrical and electronic behaviors. The simple statement "like electric charges repel each other" encapsulates a profound truth about the fundamental interactions that govern the world around us. By delving into Coulomb's Law and exploring real-world examples, we can gain a deeper appreciation of this vital aspect of physics. The accurate understanding of this principle allows for technological innovation and advancements across multiple industries. Furthermore, understanding this principle allows us to more effectively design and build electronic devices, making them more reliable and efficient. The repulsion of like charges is not just a simple concept; it is the basis of many essential technologies and natural phenomena.
Latest Posts
Latest Posts
-
Sodium Bicarbonate And Acetic Acid Reaction
Mar 17, 2025
-
What Are The Determinants Of Supply
Mar 17, 2025
-
What Percent Is 48 Out Of 60
Mar 17, 2025
-
Which Of The Following Is Are True About Natural Selection
Mar 17, 2025
-
Boiling Water Physical Or Chemical Change
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Like Electric Charges Repel Each Other. True Or False . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
