Sodium Bicarbonate And Acetic Acid Reaction
News Leon
Mar 17, 2025 · 6 min read
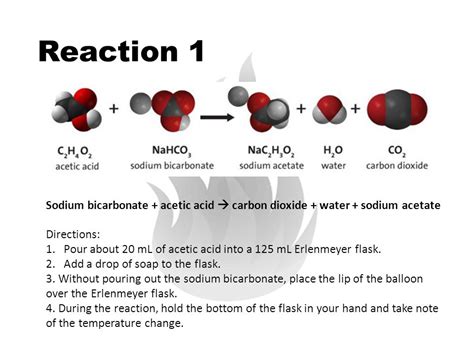
Table of Contents
The Fizz Factor: A Deep Dive into the Sodium Bicarbonate and Acetic Acid Reaction
The fizzing reaction between sodium bicarbonate (baking soda) and acetic acid (vinegar) is a common household occurrence, familiar to anyone who's ever made a baking soda volcano. But beyond the fun, this seemingly simple reaction reveals a wealth of fascinating chemical principles. This article will explore the reaction in detail, examining its chemical equation, the underlying mechanisms, its applications, and some interesting variations.
Understanding the Reaction: A Chemical Breakdown
The reaction between sodium bicarbonate (NaHCO₃) and acetic acid (CH₃COOH) is a classic example of an acid-base reaction, more specifically, an acid-base neutralization reaction. It's also a double displacement reaction, where the cations and anions of two different compounds switch places to form two new compounds.
The chemical equation representing this reaction is:
NaHCO₃(aq) + CH₃COOH(aq) → CH₃COONa(aq) + H₂O(l) + CO₂(g)
Let's break down what each component represents:
-
NaHCO₃ (Sodium Bicarbonate): This is a weak base, commonly known as baking soda. It's an amphoteric substance, meaning it can act as both an acid and a base, depending on the circumstances. In this reaction, it acts as a base.
-
CH₃COOH (Acetic Acid): This is a weak acid, the main component of vinegar. Its acidity comes from the readily available proton (H⁺) in its carboxyl group (-COOH).
-
CH₃COONa (Sodium Acetate): This is a salt formed as a product of the reaction. It's the sodium salt of acetic acid and is relatively neutral.
-
H₂O (Water): Water is another product of the neutralization reaction.
-
CO₂ (Carbon Dioxide): This is the gas responsible for the characteristic fizzing observed in the reaction. It's released as bubbles, making the mixture effervesce.
The Mechanism: A Step-by-Step Look
The reaction proceeds in several steps:
-
Proton Transfer: The acidic proton (H⁺) from the acetic acid molecule is transferred to the bicarbonate ion (HCO₃⁻) of sodium bicarbonate. This forms carbonic acid (H₂CO₃).
-
Carbonic Acid Decomposition: Carbonic acid is unstable and readily decomposes into water (H₂O) and carbon dioxide (CO₂). This decomposition is the source of the gas bubbles.
-
Salt Formation: The remaining acetate ion (CH₃COO⁻) from the acetic acid and the sodium ion (Na⁺) from the sodium bicarbonate combine to form sodium acetate (CH₃COONa), which remains dissolved in the solution.
The overall reaction is essentially a fast, exothermic process, meaning it releases heat. However, the amount of heat released is relatively small and generally not noticeable unless conducted on a large scale.
Factors Affecting the Reaction Rate
Several factors can influence the rate at which the reaction proceeds:
-
Concentration: Increasing the concentration of either the acetic acid or sodium bicarbonate will increase the reaction rate. A higher concentration means more reactant molecules are available to collide and react.
-
Temperature: Increasing the temperature generally increases the reaction rate. Higher temperatures provide the reacting molecules with more kinetic energy, leading to more frequent and successful collisions.
-
Surface Area: For solid sodium bicarbonate, increasing its surface area (e.g., by using finely powdered baking soda) will accelerate the reaction. This is because a larger surface area provides more contact points for the acetic acid to react with.
-
Presence of Catalysts: While not commonly used in household applications, certain catalysts could theoretically accelerate the reaction rate. However, this aspect is rarely explored in typical demonstrations.
Applications of the Reaction: Beyond the Volcano
The reaction between sodium bicarbonate and acetic acid, seemingly simple, finds surprisingly diverse applications:
-
Baking: The leavening action in many baked goods relies on this reaction. Baking soda reacts with acidic ingredients in the recipe (like buttermilk or lemon juice) to produce carbon dioxide gas, which creates air pockets and makes the baked goods rise.
-
Cleaning: The fizzing action can be used for cleaning purposes. The reaction helps to loosen dirt and grime, making it easier to remove. It's often used in cleaning solutions for removing stains and odors.
-
Antacids: Sodium bicarbonate is a common component of antacids. It neutralizes excess stomach acid, providing relief from heartburn and indigestion. The reaction with stomach acid (primarily hydrochloric acid) is similar to its reaction with acetic acid.
-
Fire Extinguishers: Some fire extinguishers utilize sodium bicarbonate to extinguish fires. The carbon dioxide released smothers the flames, preventing further combustion. This application mainly utilizes sodium bicarbonate's ability to release carbon dioxide under heat.
-
Chemical Demonstrations: The visually striking nature of the reaction makes it an excellent demonstration in chemistry classrooms and science experiments, showcasing fundamental chemical principles in an engaging way.
Variations and Extensions: Exploring Further
The basic reaction can be modified and extended in several intriguing ways:
-
Using Different Acids: Other weak acids can be substituted for acetic acid, resulting in similar reactions but with varying reaction rates and byproduct properties. Citric acid (found in citrus fruits) or tartaric acid (found in grapes) are good examples.
-
Controlling the Reaction Rate: By carefully controlling the concentration and addition rate of the reactants, the rate of gas production can be regulated, creating interesting effects in demonstrations.
-
Investigating the Products: Experiments can be designed to analyze and identify the products of the reaction, such as sodium acetate and carbon dioxide. This allows for a deeper understanding of stoichiometry and reaction yields.
-
Exploring the pH Changes: Monitoring the pH of the solution throughout the reaction provides insights into the neutralization process and the resulting pH of the sodium acetate solution.
-
Quantitative Analysis: By carefully measuring the amounts of reactants and products, the reaction can be used to explore concepts like stoichiometry, limiting reactants, and percentage yields.
Safety Precautions: Handling with Care
While generally safe, handling chemicals requires caution:
-
Eye Protection: Always wear safety goggles when performing experiments involving chemicals.
-
Ventilation: The reaction produces carbon dioxide, which is not toxic in small amounts, but good ventilation is still recommended.
-
Proper Disposal: Dispose of the reaction mixture responsibly according to local regulations.
Conclusion: A Reaction with Lasting Impact
The seemingly simple reaction between sodium bicarbonate and acetic acid is far richer and more complex than it first appears. Its underlying chemistry, diverse applications, and potential for experimentation make it a fascinating subject for both amateur enthusiasts and seasoned chemists alike. Understanding this reaction opens doors to exploring broader concepts in chemistry, offering a practical and engaging way to learn about acid-base reactions, gas evolution, and the exciting world of chemical transformations. From baking a cake to extinguishing a fire, the fizzing reaction continues to play a significant role in our daily lives and scientific endeavors. This detailed exploration hopefully sheds light on the beauty and versatility of this ubiquitous chemical reaction.
Latest Posts
Latest Posts
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Sodium Bicarbonate And Acetic Acid Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
