Boiling Water Physical Or Chemical Change
News Leon
Mar 17, 2025 · 5 min read
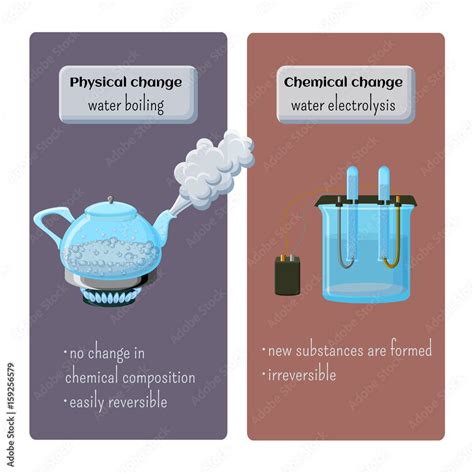
Table of Contents
Boiling Water: A Deep Dive into Physical Change
Boiling water is a ubiquitous process, yet the underlying science often remains unexplored. Is boiling water a physical or chemical change? The simple answer is physical. But understanding why requires a deeper exploration of the process, encompassing the concepts of states of matter, energy transfer, and the subtle differences between physical and chemical transformations. This article delves into the intricacies of boiling water, explaining the scientific principles involved and dispelling common misconceptions.
Understanding Physical vs. Chemical Changes
Before we dissect the boiling process, let's establish a clear understanding of the difference between physical and chemical changes. This distinction is crucial in correctly categorizing boiling water.
Physical Change: A physical change alters the form or appearance of a substance without changing its chemical composition. The substance remains the same at a molecular level. Examples include melting ice, dissolving sugar in water, or, as we'll explore in detail, boiling water.
Chemical Change: A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Evidence of a chemical change often includes a color change, the production of gas, or a change in temperature. Examples include burning wood, rusting iron, or baking a cake.
The Process of Boiling Water: A Molecular Perspective
Water, in its liquid state, consists of molecules (H₂O) held together by relatively weak intermolecular forces, primarily hydrogen bonds. These bonds allow the molecules to move freely, but still maintain some proximity to each other.
When heat is applied to water, the kinetic energy of the water molecules increases. This increased kinetic energy causes the molecules to vibrate and move more rapidly. As the temperature rises, the intermolecular forces are gradually overcome.
The Boiling Point: The boiling point is the temperature at which the vapor pressure of the liquid equals the external pressure. At sea level, the boiling point of water is 100°C (212°F). At this point, the kinetic energy of the water molecules is sufficient to overcome the intermolecular forces completely. This leads to a rapid transition from the liquid phase to the gaseous phase (steam).
Phase Transition: Boiling is a phase transition, a change from one state of matter to another. In this instance, it's a transition from liquid water to gaseous water (water vapor or steam). Crucially, the chemical composition of the water remains unchanged. It's still H₂O molecules; only their arrangement and energy levels have altered.
Observable Changes During Boiling
Several observable changes accompany the boiling of water, reinforcing its classification as a physical change:
-
Temperature remains constant: Once boiling begins, the temperature of the water remains relatively constant at 100°C (at sea level) until all the water has evaporated. This is because the added energy is used to overcome the intermolecular forces and change the state, not to increase the kinetic energy of the molecules further.
-
Formation of bubbles: The bubbles observed during boiling are pockets of water vapor that form within the liquid. These bubbles rise to the surface and escape into the atmosphere, indicating the transformation of liquid water into gaseous water.
-
Volume increase: The volume of water significantly increases upon boiling because the gas phase occupies a much larger volume than the liquid phase. This is a consequence of the increased distance between water molecules in the gaseous state.
Factors Affecting Boiling Point
While the boiling point of water is typically 100°C at sea level, several factors can influence this temperature:
-
Altitude: At higher altitudes, atmospheric pressure is lower. This means the vapor pressure of water needs to reach a lower value to equal the external pressure, resulting in a lower boiling point. Water boils at a lower temperature on mountaintops than at sea level.
-
Impurities: Dissolved substances in water can affect its boiling point. The presence of salts or other solutes generally raises the boiling point, a phenomenon known as boiling point elevation. This is because the solute particles interfere with the escape of water molecules from the liquid surface.
-
Pressure: Increasing the external pressure on the water increases its boiling point. Conversely, decreasing the pressure lowers the boiling point. Pressure cookers utilize this principle to cook food faster at higher temperatures.
Dispelling Common Misconceptions
Several misconceptions surround the boiling of water:
-
Boiling purifies water: While boiling can kill harmful microorganisms, it doesn't remove dissolved minerals or other impurities. To purify water completely, more advanced techniques such as distillation or filtration are necessary.
-
Steam is hotter than boiling water: While steam can be hotter than boiling water, it's not inherently so. Steam at 100°C has the same temperature as boiling water at 100°C. However, steam possesses a significant amount of latent heat, meaning it can release a large amount of heat energy as it condenses back into liquid water. This is why steam burns are often more severe than boiling water burns.
-
All liquids boil at 100°C: This is incorrect. Each substance has its own characteristic boiling point, determined by its intermolecular forces and molecular weight.
Applications of Boiling Water
Boiling water is a fundamental process with numerous applications in everyday life and various industries:
-
Cooking: Boiling water is extensively used in cooking for various purposes, such as boiling eggs, pasta, vegetables, and sterilizing utensils.
-
Cleaning: Boiling water is a highly effective cleaning agent, capable of sanitizing surfaces and removing grease and grime.
-
Sterilization: Boiling is a simple and effective method for sterilizing medical instruments and other items where microbial contamination is a concern.
-
Industrial processes: Boiling water plays a crucial role in several industrial processes, including power generation, chemical manufacturing, and food processing.
Conclusion: Boiling – A Definitive Physical Change
In conclusion, boiling water is unequivocally a physical change. While the process involves energy transfer and a visible change of state, the chemical composition of the water remains unaltered. The transition from liquid to gas is driven by the increased kinetic energy of water molecules overcoming intermolecular forces. Understanding this distinction is fundamental to comprehending the basic principles of chemistry and physics. The detailed examination of this seemingly simple process reveals a wealth of scientific principles with wide-ranging applications in various fields. This understanding helps us appreciate the seemingly simple processes that underpin our everyday lives. Furthermore, understanding the nuances of boiling helps us harness its power for various practical applications, from simple cooking to complex industrial procedures.
Latest Posts
Latest Posts
-
How Many Feet Is 1 2 Miles
Mar 18, 2025
-
How Many Valence Electrons Does Mn Have
Mar 18, 2025
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
