Is Sand And Water A Solution
News Leon
Mar 31, 2025 · 6 min read
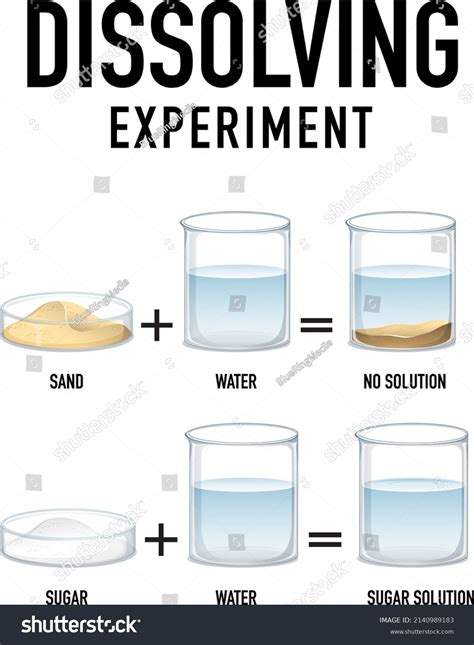
Table of Contents
Is Sand and Water a Solution? Exploring Mixtures and Their Properties
The question, "Is sand and water a solution?" seems simple, but it delves into the fundamental concepts of chemistry and the differences between various types of mixtures. The short answer is no, sand and water do not form a solution. However, understanding why requires a deeper exploration of solutions, suspensions, and the properties that distinguish them. This article will thoroughly examine this seemingly simple question, providing a comprehensive understanding of mixtures and their characteristics.
Understanding Solutions, Suspensions, and Colloids
Before we delve into the specifics of sand and water, let's establish a clear understanding of different types of mixtures. A mixture is a combination of two or more substances where the individual substances retain their chemical identities. Crucially, mixtures can be separated by physical means, unlike compounds which require chemical reactions for separation. Within mixtures, we find three main categories:
1. Solutions: A Homogeneous Blend
A solution is a homogeneous mixture, meaning its composition is uniform throughout. It consists of a solute, the substance being dissolved, and a solvent, the substance doing the dissolving. The solute particles are dispersed at a molecular level within the solvent, resulting in a transparent or translucent mixture. Think of saltwater: the salt (solute) dissolves completely in the water (solvent), creating a uniform mixture. Key characteristics of solutions include:
- Homogeneity: Uniform composition throughout.
- Particle size: Solute particles are at the atomic or molecular level (less than 1 nm).
- Transparency: Solutions are typically transparent or translucent.
- Filtration: Solute particles cannot be separated by filtration.
2. Suspensions: A Heterogeneous Mixture
A suspension is a heterogeneous mixture, meaning its composition is not uniform. The particles of the dispersed substance are larger than those in a solution and are not completely dissolved. These particles will settle out over time if left undisturbed. Think of muddy water: the mud particles (solute) are suspended in the water (solvent), but they are visibly separate and will settle to the bottom if the mixture is left standing. Characteristics of suspensions include:
- Heterogeneity: Non-uniform composition.
- Particle size: Solute particles are relatively large (greater than 1000 nm).
- Opacity: Suspensions are usually opaque.
- Filtration: Solute particles can be separated by filtration.
- Sedimentation: Particles settle out over time.
3. Colloids: In Between Solutions and Suspensions
Colloids occupy a middle ground between solutions and suspensions. They are heterogeneous mixtures where the dispersed particles are larger than those in a solution but smaller than those in a suspension (between 1 nm and 1000 nm). These particles do not settle out readily. Examples include milk, fog, and mayonnaise. Colloids exhibit the Tyndall effect, scattering light, creating a cloudy appearance. Key characteristics of colloids:
- Heterogeneity: Non-uniform composition, though often appearing homogeneous.
- Particle size: Intermediate particle size (1 nm to 1000 nm).
- Opacity: Often cloudy or opaque due to light scattering.
- Filtration: Particles generally do not pass through a filter.
- Tyndall effect: Scatter light.
Why Sand and Water is a Suspension, Not a Solution
Now, let's return to our original question: Is sand and water a solution? The answer is a resounding no. Sand and water form a suspension, not a solution. This is due to several key factors:
- Particle Size: Sand particles are significantly larger than individual molecules or ions. They are far too large to be dissolved at a molecular level in water.
- Solubility: Sand is insoluble in water. This means its constituent particles (primarily silicon dioxide) do not have a strong enough affinity for water molecules to break apart and disperse uniformly.
- Heterogeneity: A mixture of sand and water is clearly heterogeneous. You can readily distinguish the sand particles from the water. The sand will settle at the bottom if left undisturbed.
- Filtration: Sand particles can be easily separated from the water using simple filtration techniques.
Further Exploring the Properties of Sand and Water Mixtures
While sand and water don't form a true solution, examining their interactions reveals several interesting properties:
Settling and Sedimentation
The sand particles in a sand and water mixture will eventually settle to the bottom due to gravity. This process is known as sedimentation. The rate of sedimentation depends on several factors, including the size and density of the sand particles, the viscosity of the water, and the presence of any other substances in the mixture.
Viscosity
The viscosity, or resistance to flow, of a sand and water mixture is higher than that of pure water. The presence of the sand particles increases the internal friction within the mixture, making it more resistant to flow. This is especially noticeable at higher concentrations of sand.
Density
The overall density of a sand and water mixture will be higher than that of pure water. This is because sand is denser than water, so the addition of sand increases the overall mass of the mixture without a proportional increase in volume.
Applications and Implications of Sand and Water Mixtures
Understanding the properties of sand and water mixtures has practical applications in various fields:
Environmental Science: Studying Sediment Transport
The interaction of sand and water is crucial in understanding sediment transport in rivers, oceans, and other water bodies. The rate at which sand settles and the factors influencing this rate are vital for modeling erosion, deposition, and the overall geomorphology of landscapes.
Civil Engineering: Concrete Production and Soil Mechanics
The properties of sand-water mixtures play a significant role in civil engineering. For example, the appropriate ratio of sand to water in concrete is critical for achieving the desired strength and workability. Similarly, understanding the behavior of sand and water mixtures is crucial in soil mechanics, where it impacts factors like soil stability and drainage.
Water Treatment: Filtration and Purification
The ability to separate sand from water through filtration is a fundamental principle in water treatment processes. Sand filters are used extensively to remove suspended solids and other impurities from water sources.
Conclusion: A Clear Distinction
In conclusion, the answer to the question, "Is sand and water a solution?" is definitively no. Sand and water form a suspension, a heterogeneous mixture characterized by its distinct phases, larger particle size, and the ability to separate the components through simple physical means like filtration. While seemingly a simple question, understanding this distinction highlights the importance of grasping the fundamental concepts of solutions, suspensions, and colloids. This knowledge is crucial in various scientific and engineering disciplines, impacting fields like environmental science, civil engineering, and water treatment. The properties of sand and water mixtures, such as sedimentation, viscosity, and density, have profound implications for understanding natural processes and designing effective engineering solutions.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 36 And 84
Apr 02, 2025
-
An Atom With 3 Protons And 4 Neutrons
Apr 02, 2025
-
Which Of The Following Contains An Example Of Alliteration
Apr 02, 2025
-
All Of The Following Characteristics Are Associated With Epithelium Except
Apr 02, 2025
-
This Organelle Pumps Out Excess Water
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Sand And Water A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
