Is Boil A Physical Or Chemical Change
News Leon
Mar 28, 2025 · 5 min read
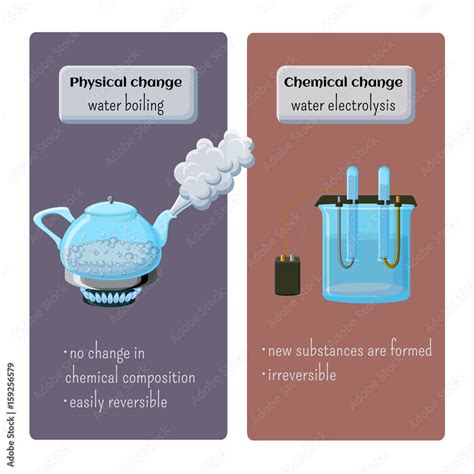
Table of Contents
Is Boiling a Physical or Chemical Change? A Deep Dive
The question of whether boiling is a physical or chemical change is a common one, often sparking debate among students and science enthusiasts alike. While seemingly straightforward, a complete understanding requires delving into the fundamental principles of matter and its transformations. This comprehensive article will explore the intricacies of boiling, examining the evidence to definitively answer this question and clarifying the distinctions between physical and chemical changes.
Understanding Physical and Chemical Changes
Before we tackle the boiling point, let's establish a clear understanding of the difference between physical and chemical changes. These two types of changes represent distinct transformations of matter:
Physical Changes:
- Definition: A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same chemically; only its physical properties (like shape, size, or state) are modified.
- Examples: Melting ice, dissolving sugar in water, cutting paper, boiling water. In each case, the chemical identity of the substance remains unchanged.
- Reversibility: Many physical changes are reversible. For example, you can freeze liquid water back into ice.
Chemical Changes:
- Definition: A chemical change involves a rearrangement of atoms and molecules, resulting in the formation of new substances with different chemical properties. The original substance is fundamentally transformed.
- Examples: Burning wood, rusting iron, cooking an egg. These processes create entirely new substances with different properties.
- Irreversibility: Chemical changes are generally irreversible. You cannot easily turn the ashes from burned wood back into the original wood.
The Boiling Process: A Detailed Examination
Boiling is a phase transition, specifically the transformation of a liquid into a gas. Let's dissect the process step-by-step:
The Role of Heat Energy:
Boiling requires the input of heat energy. This energy increases the kinetic energy of the liquid's molecules, causing them to move more rapidly and vigorously. As the temperature rises, the intermolecular forces holding the molecules together weaken.
Vapor Pressure and Atmospheric Pressure:
The boiling point is reached when the vapor pressure of the liquid equals the atmospheric pressure. Vapor pressure is the pressure exerted by the vapor molecules above the liquid. At sea level, atmospheric pressure is relatively high, requiring a higher temperature to reach the boiling point. At higher altitudes, where atmospheric pressure is lower, the boiling point decreases.
Formation of Bubbles:
As the liquid reaches its boiling point, vapor bubbles begin to form within the liquid. These bubbles consist of the liquid's vapor (its gaseous phase). They rise to the surface and burst, releasing the vapor into the atmosphere. This is the characteristic bubbling associated with boiling.
Evidence Supporting Boiling as a Physical Change:
Despite the dramatic appearance of boiling, numerous observations support its classification as a physical change:
-
No New Substance is Formed: When water boils, it transforms from liquid water (H₂O) to gaseous water (water vapor or steam), which is still H₂O. The chemical formula remains unchanged. This is the defining characteristic of a physical change.
-
Reversibility: The process of boiling is reversible through condensation. Water vapor can be cooled, causing it to revert back to its liquid state. This reversibility is a strong indicator of a physical change.
-
Separation Techniques: Distillation, a process that relies on the boiling and condensation of liquids, is used to separate mixtures. This demonstrates that boiling doesn't alter the chemical composition of the substances being separated.
-
Chemical Properties Remain Unchanged: The chemical properties of water, such as its ability to react with certain substances, remain identical before and after boiling. If a chemical change had occurred, these properties would be altered.
Addressing Potential Misconceptions:
Several factors might seem to suggest a chemical change during boiling, but closer examination clarifies that these are merely aspects of the physical process:
- Bubbles: The formation of bubbles is a visual phenomenon related to the change in state, not a sign of a new substance forming.
- Changes in Appearance: The transition from liquid to gas changes the appearance of water, but this is a superficial change, not a fundamental alteration in its chemical structure.
- Energy Transfer: The absorption of heat energy is necessary for the phase change but doesn't signify a chemical reaction. Energy is absorbed to overcome the intermolecular forces holding the water molecules together.
Boiling vs. Decomposition: A Crucial Distinction
It's important to distinguish boiling from decomposition. Decomposition is a chemical change where a substance breaks down into simpler substances. For example, heating certain carbonates can lead to their decomposition into oxides and carbon dioxide. This is fundamentally different from boiling, where the substance remains chemically the same.
Conclusion: Boiling is a Physical Change
The overwhelming evidence points to boiling as a physical change. While it involves a dramatic change in state and appearance, the fundamental chemical composition of the substance remains unchanged. The reversibility of the process, the absence of new substance formation, and the preservation of chemical properties all support this classification. Understanding this distinction is crucial for grasping the fundamental principles of matter and its transformations.
Keywords for SEO:
Boiling, physical change, chemical change, phase transition, water, vapor, steam, condensation, distillation, vapor pressure, atmospheric pressure, kinetic energy, intermolecular forces, decomposition, reversible, irreversible, H2O, scientific explanation, science, chemistry, physics.
Semantic Keywords:
State change, matter transformation, molecular movement, heat energy transfer, boiling point, changes of state, properties of matter, scientific method, experimental observation, scientific analysis, evidence-based reasoning.
Long-Tail Keywords:
Is boiling water a physical or chemical change and why?, what happens to water molecules when they boil?, explain the difference between boiling and a chemical reaction, is boiling water reversible, how does boiling relate to vapor pressure, how does altitude affect boiling point, difference between boiling and decomposition.
Latest Posts
Latest Posts
-
A Person Who Study History Is Called
Mar 31, 2025
-
Is Calcium Oxide Ionic Or Covalent
Mar 31, 2025
-
What Is Not True Regarding Antibiotics
Mar 31, 2025
-
Balanced Equation For Copper And Nitric Acid
Mar 31, 2025
-
Passwords Passphrases And Pins Are Examples Of Which Security Term
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Boil A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
