Balanced Equation For Copper And Nitric Acid
News Leon
Mar 31, 2025 · 5 min read
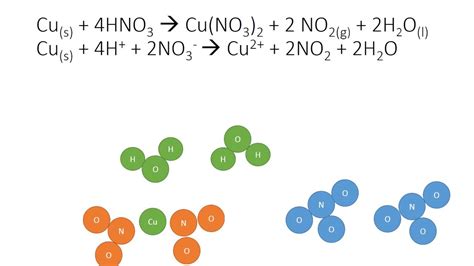
Table of Contents
The Balanced Equation for Copper and Nitric Acid: A Deep Dive into Redox Chemistry
The reaction between copper metal (Cu) and nitric acid (HNO₃) is a classic example of a redox reaction, a chemical process involving the transfer of electrons between species. This reaction is widely studied in chemistry education for its fascinating aspects and its implications in various industrial processes. Understanding the balanced equation, however, requires careful consideration of the reaction conditions and the possible products formed. This article will explore the intricacies of this reaction, providing a comprehensive understanding of the balanced equations under different conditions, including the underlying redox chemistry and practical applications.
Understanding the Fundamentals: Redox Reactions
Before delving into the specifics of the copper-nitric acid reaction, let's establish a strong foundation in redox chemistry. A redox reaction, or oxidation-reduction reaction, is characterized by the simultaneous occurrence of oxidation and reduction.
- Oxidation: The loss of electrons by a species. The oxidation state of the species increases.
- Reduction: The gain of electrons by a species. The oxidation state of the species decreases.
A useful mnemonic device to remember this is OIL RIG: Oxidation Is Loss, Reduction Is Gain (of electrons).
In any redox reaction, there must be a species that is oxidized (the reducing agent) and a species that is reduced (the oxidizing agent). These two processes are always coupled; one cannot occur without the other.
The Reaction of Copper and Nitric Acid: A Multifaceted Process
The reaction between copper and nitric acid is complex because it yields different products depending on the concentration of the nitric acid. Concentrated nitric acid (typically 16 M) yields nitrogen dioxide (NO₂), while dilute nitric acid (typically around 6 M) produces nitric oxide (NO). Let's explore each case individually.
Reaction 1: Copper and Concentrated Nitric Acid
When copper reacts with concentrated nitric acid, the balanced equation is:
Cu(s) + 4HNO₃(conc.) → Cu(NO₃)₂(aq) + 2NO₂(g) + 2H₂O(l)
Let's break this down:
- Cu(s): Copper in its solid metallic state, acting as the reducing agent.
- 4HNO₃(conc.): Concentrated nitric acid, acting as the oxidizing agent. The high concentration favors the formation of nitrogen dioxide.
- Cu(NO₃)₂(aq): Copper(II) nitrate, a soluble salt formed in the aqueous solution.
- 2NO₂(g): Nitrogen dioxide, a reddish-brown gas.
- 2H₂O(l): Water, formed as a byproduct.
Half-Reactions: To illustrate the electron transfer, we can separate the overall reaction into two half-reactions:
- Oxidation half-reaction: Cu(s) → Cu²⁺(aq) + 2e⁻
- Reduction half-reaction: 2HNO₃(conc.) + 2e⁻ → 2NO₂(g) + H₂O(l)
This clearly shows that copper loses two electrons (oxidation) and nitric acid gains those electrons (reduction).
Reaction 2: Copper and Dilute Nitric Acid
In contrast to concentrated nitric acid, dilute nitric acid reacts with copper to produce nitric oxide (NO) instead of nitrogen dioxide (NO₂). The balanced equation for this reaction is:
3Cu(s) + 8HNO₃(dil.) → 3Cu(NO₃)₂(aq) + 2NO(g) + 4H₂O(l)
Notice the different stoichiometry compared to the reaction with concentrated nitric acid.
- 3Cu(s): Three moles of copper are required to react completely.
- 8HNO₃(dil.): Eight moles of dilute nitric acid are used as the oxidizing agent. The lower concentration favors the formation of nitric oxide.
- 3Cu(NO₃)₂(aq): Three moles of copper(II) nitrate are formed.
- 2NO(g): Two moles of nitric oxide, a colorless gas, are produced.
- 4H₂O(l): Four moles of water are produced as a byproduct.
Half-Reactions: The half-reactions for this reaction are:
- Oxidation half-reaction: 3Cu(s) → 3Cu²⁺(aq) + 6e⁻
- Reduction half-reaction: 4HNO₃(dil.) + 6e⁻ → 2NO(g) + 4H₂O(l)
Again, the half-reactions highlight the electron transfer, showcasing copper's oxidation and nitric acid's reduction.
Observational Differences and Practical Implications
The differences in the products (NO₂ vs. NO) lead to observable differences in the reactions. The reaction with concentrated nitric acid is characterized by the evolution of a dense, reddish-brown gas (NO₂), while the reaction with dilute nitric acid produces a colorless gas (NO) which quickly oxidizes in air to form brown NO₂.
These reactions have significant practical implications:
- Industrial Applications: The reaction is used in the extraction and purification of copper from its ores. The process involves dissolving copper in nitric acid, followed by further steps to recover the pure copper.
- Laboratory Use: The reaction is commonly performed in chemistry laboratories to demonstrate redox reactions and the effect of concentration on reaction products.
- Environmental Concerns: The gaseous products (NO and NO₂) are air pollutants and contribute to acid rain. Proper handling and disposal of the reaction products are essential to minimize environmental impact.
Beyond the Basics: Exploring Competing Reactions
While the reactions described above are the dominant pathways, it's important to note that other reactions can occur to a lesser extent, especially under specific conditions. These may include the formation of other nitrogen oxides (N₂O, N₂O₃, N₂O₄), or even the reduction of nitrate to ammonium ions (NH₄⁺) under highly reducing conditions. The precise product distribution is sensitive to factors like temperature, concentration, and the presence of other reactants or catalysts.
Further Exploration and Conclusion
The reaction between copper and nitric acid is a rich area of study in chemistry, illustrating the fascinating interplay of redox chemistry and reaction conditions. By understanding the balanced equations for both concentrated and dilute nitric acid, we gain insights into the fundamental principles governing these chemical transformations. This knowledge is not only crucial for academic understanding but also holds relevance in various industrial processes and environmental considerations. Further exploration of this topic might involve investigating the kinetics of the reaction, the influence of catalysts, and the development of more efficient and environmentally friendly methods for copper processing. Through continued research and deeper understanding, we can refine our knowledge and optimize the applications of this classic chemical reaction. The reaction serves as a powerful reminder of the complex and often unpredictable nature of chemical reactivity, offering endless opportunities for further scientific investigation and technological advancement.
Latest Posts
Latest Posts
-
How Many Chromosomes In Liver Cells
Apr 01, 2025
-
All Of The Following Refer To Mitosis Except
Apr 01, 2025
-
Which Of The Following Temperatures Is The Coldest
Apr 01, 2025
-
A Short Term Unsecured Promissory Note Issued By A Company Is
Apr 01, 2025
-
Adjacent Angles Whose Sum In 180 Degrees
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Copper And Nitric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
