Is A Singal Bond Stronger Than Pi
News Leon
Mar 30, 2025 · 5 min read
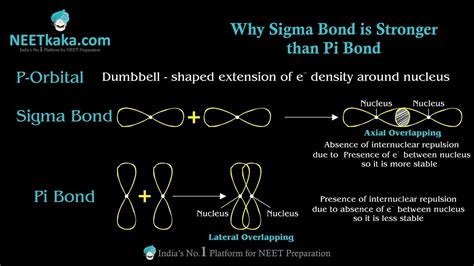
Table of Contents
Is a Single Bond Stronger Than a Pi Bond? Understanding Chemical Bonding
The question of whether a single bond is stronger than a pi bond is not a simple yes or no answer. The strength of a chemical bond is a complex issue depending on several factors, and a direct comparison often misses the nuances. This article will delve deep into the nature of single and pi bonds, exploring their strengths, weaknesses, and the circumstances under which one might be considered "stronger" than the other.
Understanding Single Bonds (Sigma Bonds)
A single bond, also known as a sigma (σ) bond, is the strongest type of covalent bond. It's formed by the head-on overlap of atomic orbitals. This direct overlap results in a high electron density concentrated between the two bonded nuclei. This strong concentration of electron density leads to a strong electrostatic attraction between the nuclei and the shared electrons, resulting in a stable bond.
Characteristics of Single Bonds:
- Strongest type of covalent bond: Due to the direct and efficient overlap of orbitals.
- Rotational freedom: Atoms connected by a single bond can rotate freely around the bond axis.
- Higher bond energy: Generally, single bonds possess higher bond energy compared to pi bonds (though this is not always the case, as we'll explore later).
- Longer bond length: Single bonds are typically longer than pi bonds because the electron density is more spread out.
Understanding Pi Bonds
A pi (π) bond is a type of covalent bond formed by the sideways or lateral overlap of p orbitals. Unlike sigma bonds, the electron density in a pi bond is concentrated above and below the internuclear axis, rather than directly between the nuclei. This less direct overlap results in a weaker bond compared to a sigma bond.
Characteristics of Pi Bonds:
- Weaker than sigma bonds: Due to less effective orbital overlap.
- No rotational freedom: The sideways overlap restricts rotation around the bond axis, leading to restricted conformation.
- Shorter bond length: Generally shorter than single bonds because the electron density is concentrated in a smaller region.
- Often found alongside sigma bonds: Pi bonds rarely exist independently; they usually accompany a sigma bond to form double or triple bonds.
Comparing Bond Strength: Bond Energy and Bond Length
The strength of a bond is typically measured by its bond energy, which is the energy required to break the bond. A higher bond energy indicates a stronger bond. Bond length, the distance between the nuclei of two bonded atoms, is inversely related to bond strength – shorter bonds are generally stronger.
While a single sigma bond generally possesses higher bond energy than a single pi bond, this isn't a universally applicable rule. The comparison becomes more complex when considering double and triple bonds which incorporate both sigma and pi bonds.
Double and Triple Bonds: A Combined Approach
Double bonds consist of one sigma bond and one pi bond, while triple bonds consist of one sigma bond and two pi bonds. The overall strength of a double or triple bond is greater than a single bond due to the cumulative effect of the sigma and pi bonds. However, the individual contribution of each pi bond is less than that of the sigma bond.
Example: Comparing C-C Bonds
Let's compare the different types of carbon-carbon bonds:
- Single bond (C-C): Relatively long bond length, high bond energy, free rotation.
- Double bond (C=C): Shorter bond length than a single bond, higher bond energy than a single bond (due to both sigma and pi bonds), restricted rotation.
- Triple bond (C≡C): Shortest bond length, highest bond energy, restricted rotation.
In this case, the triple bond is the strongest, followed by the double bond, and then the single bond. While each pi bond is individually weaker than the sigma bond, the collective effect of the additional pi bond(s) increases the overall bond strength and decreases the bond length.
Factors Influencing Bond Strength Beyond Bond Type
Several factors can influence the relative strength of single and pi bonds beyond their inherent differences:
- Hybridization: The hybridization of the atomic orbitals involved significantly impacts bond strength. For example, sp hybridized orbitals form stronger sigma bonds than sp2 or sp3 hybridized orbitals. This is because sp hybridized orbitals have more s character, resulting in a more compact and stronger overlap.
- Electronegativity: The difference in electronegativity between the bonded atoms can affect bond strength. A greater difference in electronegativity can lead to a more polar bond, which might be stronger or weaker depending on the specific atoms involved.
- Steric hindrance: The presence of bulky substituent groups can cause steric hindrance, weakening the bond by affecting optimal orbital overlap.
- Resonance: In molecules with resonance structures, delocalization of electrons can strengthen bonds by distributing electron density over multiple atoms.
Conclusion: Context Matters
The simple statement "a single bond is stronger than a pi bond" is an oversimplification. While a sigma bond is inherently stronger than a single pi bond due to its direct head-on overlap, the overall strength of a bond is a complex interplay of factors. When considering double and triple bonds, the cumulative strength of multiple sigma and pi bonds significantly increases the overall bond strength compared to a single sigma bond.
Ultimately, determining whether a specific single bond is "stronger" than a specific pi bond requires considering the specific atoms involved, their hybridization, the overall molecular structure, and other influencing factors. The context is critical in making a meaningful comparison.
Latest Posts
Latest Posts
-
Above The Critical Temperature A Substance
Apr 01, 2025
-
Is Tarnish A Physical Or Chemical Change
Apr 01, 2025
-
How Many Valence Electrons Does Each Atom Of Arsenic Have
Apr 01, 2025
-
If I Drink I Die Riddle
Apr 01, 2025
-
Draw And Label A Ph Scale
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is A Singal Bond Stronger Than Pi . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
