Above The Critical Temperature A Substance
News Leon
Apr 01, 2025 · 5 min read
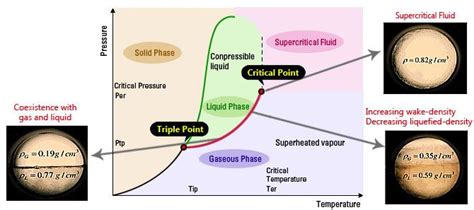
Table of Contents
Above the Critical Temperature: A Deep Dive into Supercritical Fluids
Understanding the behavior of substances above their critical temperature is crucial across various scientific and engineering disciplines. This state, known as the supercritical fluid (SCF) state, possesses unique properties that make it invaluable in numerous applications, from enhanced oil recovery to pharmaceutical processing. This article will delve into the intricacies of supercritical fluids, exploring their characteristics, behaviors, and the significance of exceeding the critical temperature.
What is the Critical Point?
Before understanding supercritical fluids, we must define the critical point. This point represents the end of the liquid-vapor coexistence curve on a phase diagram. It's characterized by specific values of critical temperature (Tc) and critical pressure (Pc). Above these values, the distinction between liquid and gas phases disappears. The critical point is not merely a point on a graph; it signifies a fundamental change in the nature of a substance's behavior.
Understanding Phase Diagrams
Phase diagrams are essential tools for visualizing the state of a substance under varying conditions of temperature and pressure. These diagrams illustrate the different phases (solid, liquid, gas) a substance can exist in and the transitions between them. The critical point is a crucial feature of these diagrams, marking the boundary beyond which the liquid and gas phases become indistinguishable.
Critical Temperature's Significance
The critical temperature (Tc) is the temperature above which a substance cannot exist as a liquid, regardless of the applied pressure. This is a fundamental property of a substance, determined by the strength of its intermolecular forces. Substances with strong intermolecular forces have higher critical temperatures.
The Unique Properties of Supercritical Fluids
Above the critical temperature and critical pressure, a substance exists as a supercritical fluid (SCF). These fluids exhibit remarkable properties that set them apart from both liquids and gases:
- Density: SCFs possess densities comparable to liquids, facilitating efficient dissolution and extraction processes.
- Diffusivity: SCF diffusivities are closer to gases, resulting in faster mass transfer rates. This is advantageous in many applications requiring rapid penetration and reaction.
- Viscosity: The viscosity of SCFs lies between that of liquids and gases, enabling them to penetrate porous materials effectively while maintaining reasonable flow rates.
- Solubility: SCF solubility is highly tunable. By adjusting pressure and temperature, one can precisely control the solubility of various compounds within the SCF, making them excellent solvents for selective extractions.
Applications of Supercritical Fluids
The unique properties of SCFs have led to their widespread use in various industries and research fields:
1. Supercritical Fluid Extraction (SFE)
SFE is a powerful technique for isolating and purifying valuable compounds from complex matrices. Supercritical CO2 (SC-CO2), in particular, is a popular choice due to its low critical temperature (31.1 °C), low toxicity, and readily available nature. SFE is employed in:
- Food industry: decaffeination of coffee and tea, extraction of essential oils and flavors from spices and herbs.
- Pharmaceutical industry: extraction of active compounds from natural products, purification of pharmaceuticals.
- Environmental remediation: extraction of pollutants from contaminated soil and water.
2. Supercritical Fluid Chromatography (SFC)
SFC is an analytical technique that utilizes SCFs as the mobile phase. It combines the advantages of both gas chromatography (GC) and high-performance liquid chromatography (HPLC), offering enhanced separation capabilities for a wide range of compounds. SFC is used in:
- Analysis of pharmaceuticals: separation and quantification of drug components and impurities.
- Environmental analysis: detection and quantification of pollutants in environmental samples.
- Food analysis: identification and quantification of food components and additives.
3. Particle Engineering
SCFs are used to create nano- and micro-particles with precisely controlled size and morphology. This technique, known as supercritical antisolvent precipitation (SAS), utilizes SCFs to rapidly precipitate dissolved compounds, resulting in fine particles with enhanced properties. Applications include:
- Pharmaceutical industry: production of drug delivery systems with improved bioavailability.
- Materials science: synthesis of nanomaterials with tailored properties.
- Cosmetics industry: production of microparticles for controlled release formulations.
4. Enhanced Oil Recovery (EOR)
Injecting SC-CO2 into oil reservoirs can improve oil recovery by reducing the viscosity of the oil and increasing its mobility. This technique is particularly effective in mature oil fields where conventional methods have become less efficient.
5. Chemical Reactions
SCFs can act as reaction media for various chemical transformations. Their tunable properties allow for precise control over reaction kinetics and selectivity, leading to improved yields and reduced byproduct formation.
Advantages of Using Supercritical Fluids
The adoption of SCFs offers several significant advantages:
- Reduced environmental impact: SCFs often eliminate the need for harmful organic solvents, contributing to greener chemical processes.
- High efficiency: The unique properties of SCFs lead to faster reaction rates and more efficient separations.
- Improved product quality: SCFs can produce products with higher purity and improved properties.
- Cost-effectiveness: In some cases, SCF processes can be more cost-effective than traditional methods.
Limitations of Using Supercritical Fluids
Despite their many advantages, SCFs also present some challenges:
- High pressure requirements: Maintaining the SCF state necessitates high-pressure equipment, which can increase capital costs and safety concerns.
- Specialized equipment: Working with SCFs requires specialized equipment and expertise.
- Solubility limitations: While SCF solubility is tunable, it may not be suitable for all compounds.
- Safety concerns: High pressures involved necessitate rigorous safety protocols and specialized training.
Future Trends in Supercritical Fluid Technology
Research and development in SCF technology continue to advance, leading to several promising future trends:
- Novel applications: Researchers are continuously exploring new applications of SCFs, expanding their use in diverse fields.
- Improved equipment: Advancements in equipment design are addressing the challenges associated with high-pressure operation.
- Process optimization: Researchers are focusing on optimizing SCF processes to improve efficiency and reduce costs.
- Sustainable practices: Emphasis is placed on developing more environmentally benign SCF processes.
Conclusion
Supercritical fluids represent a powerful technology with a wide range of applications across numerous industries. Understanding the behavior of substances above their critical temperature is essential for harnessing the unique properties of SCFs and advancing their use in various fields. While challenges remain, ongoing research and development promise to further expand the potential of this versatile technology, contributing to greener and more efficient processes in the future. The ability to precisely control solubility, diffusivity, and other properties by manipulating pressure and temperature makes supercritical fluids an invaluable tool for scientists and engineers seeking innovative solutions across diverse sectors. Their use continues to grow, driven by both economic and environmental benefits, solidifying their position as a cornerstone of modern chemistry and engineering.
Latest Posts
Latest Posts
-
Hyposecretion Of The Thyroid Gland In Adulthood
Apr 02, 2025
-
At What Temperature Do The Celsius And Fahrenheit Scales Coincide
Apr 02, 2025
-
2 Is The Only Even Prime Number
Apr 02, 2025
-
Distance Between Earth And Moon In Light Years
Apr 02, 2025
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Above The Critical Temperature A Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
