How Many Valence Electrons Does Each Atom Of Arsenic Have
News Leon
Apr 01, 2025 · 6 min read
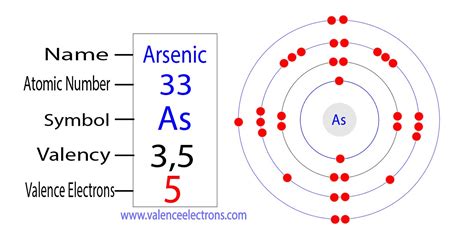
Table of Contents
How Many Valence Electrons Does Each Atom of Arsenic Have? A Deep Dive into Arsenic's Electronic Structure
Arsenic, a metalloid element with the symbol As and atomic number 33, plays a fascinating role in various fields, from semiconductor technology to its controversial use in pesticides. Understanding its chemical behavior requires a firm grasp of its electronic structure, specifically the number of valence electrons it possesses. This article will delve deep into the answer to the central question: how many valence electrons does each atom of arsenic have? We'll explore the concept of valence electrons, arsenic's position on the periodic table, its electron configuration, and the implications of its valence electrons for its chemical reactivity.
Understanding Valence Electrons
Before we pinpoint arsenic's valence electron count, let's clarify what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely bound to the nucleus and are therefore the ones most involved in chemical bonding. They determine an element's chemical properties and its ability to form bonds with other atoms. The number of valence electrons dictates the atom's reactivity and the type of bonds it can form (ionic, covalent, metallic). Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often resembling the noble gases. This is often referred to as the octet rule, although it has exceptions, especially for elements beyond the third period.
Arsenic's Position on the Periodic Table
The periodic table organizes elements based on their atomic structure and properties. Arsenic is located in Group 15 (or VA), also known as the pnictogens. This group is characterized by elements having five valence electrons. This position provides a crucial clue in determining arsenic's valence electron count. Elements within the same group tend to exhibit similar chemical properties due to their identical number of valence electrons.
Arsenic's Electron Configuration
The electron configuration of an atom describes how electrons are distributed among its various energy levels and sublevels. Arsenic's electron configuration is [Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>3</sup>. Let's break this down:
- [Ar]: This represents the electron configuration of Argon (1s²2s²2p⁶3s²3p⁶), a noble gas. This core configuration signifies that arsenic's inner electrons are arranged similarly to Argon.
- 3d<sup>10</sup>: Ten electrons fill the 3d sublevel. These are inner electrons and are not considered valence electrons.
- 4s<sup>2</sup>: Two electrons occupy the 4s sublevel.
- 4p<sup>3</sup>: Three electrons reside in the 4p sublevel.
The crucial point here is that the outermost shell (n=4) contains the 4s and 4p electrons. Therefore, the total number of valence electrons in an arsenic atom is 2 (from 4s) + 3 (from 4p) = 5.
The Significance of Arsenic's Five Valence Electrons
The presence of five valence electrons significantly influences arsenic's chemical behavior:
- Covalent Bonding: Arsenic readily forms covalent bonds, sharing its valence electrons with other atoms to achieve a more stable configuration. This is evident in compounds like arsenic trioxide (As₂O₃) and arsenic pentoxide (As₂O₅).
- Variable Oxidation States: Because of its five valence electrons, arsenic can exhibit variable oxidation states, ranging from -3 to +5. This means it can either gain three electrons to reach a full outer shell, or lose three or five electrons depending on the chemical environment.
- Semiconductor Properties: The electronic structure of arsenic contributes to its semiconducting properties. Arsenic is a vital component in various semiconductor materials used in electronic devices. The ability of arsenic to share or accept electrons is crucial for its function in these materials.
- Toxicity: The chemical reactivity of arsenic, stemming from its valence electrons, is directly linked to its toxicity. Arsenic compounds can interfere with various biochemical processes in living organisms.
Arsenic's Role in Different Fields
The unique properties of arsenic, largely determined by its five valence electrons, make it relevant in various applications:
1. Semiconductor Industry:
Arsenic is a crucial dopant in semiconductor materials like silicon and gallium arsenide (GaAs). Doping involves introducing impurities to alter the material's electrical conductivity. Arsenic's ability to easily share or accept electrons makes it effective in creating either n-type or p-type semiconductors, essential components in transistors, integrated circuits, and other electronic devices. The precise control of arsenic doping is vital for achieving the desired electronic properties.
2. Medicine:
Historically, arsenic compounds have been used in medicine, though their toxicity necessitates careful control and modern alternatives are often preferred. Some arsenic compounds have shown promise in treating certain cancers, although research continues to refine their application and understand their mechanisms of action.
3. Agriculture:
Despite its toxicity, arsenic compounds have been used in the past as pesticides and herbicides. However, due to environmental and health concerns, their use is now heavily regulated or banned in many regions. The persistent nature of arsenic in the environment poses significant challenges for remediation efforts.
4. Metallurgy:
Arsenic is sometimes found as an impurity in metal ores and can affect the properties of the resulting metals. In some cases, it might be deliberately added to alloys to enhance their properties, although this is less common than other alloying elements.
Comparing Arsenic with Other Group 15 Elements
Arsenic shares many similarities with other elements in Group 15 (nitrogen, phosphorus, antimony, bismuth). All these elements have five valence electrons and exhibit some similar chemical behaviors. However, there are also significant differences:
- Nitrogen and Phosphorus: These elements are nonmetals and typically form covalent bonds more readily than arsenic.
- Antimony and Bismuth: These are also metalloids, but their metallic character is more pronounced than arsenic's. They are less reactive than arsenic.
The variations in reactivity and properties among Group 15 elements stem from differences in atomic size, electronegativity, and the influence of d-orbitals in heavier elements. While all share the defining feature of five valence electrons, the specific manifestation of this feature varies significantly across the group.
Conclusion: Arsenic's Five Valence Electrons: A Cornerstone of its Properties
In conclusion, each atom of arsenic possesses five valence electrons. This seemingly simple fact is fundamental to understanding arsenic's chemical reactivity, its various applications, and its potential environmental impacts. From its role in the semiconductor industry to its historical (and controversial) use in medicine and agriculture, arsenic's behavior is fundamentally shaped by its electronic structure. A thorough understanding of its valence electrons is crucial for harnessing its useful properties while mitigating its potential toxicity. Further research continues to unravel the complexities of arsenic's behavior, revealing its unique place within the periodic table and the broader world of chemistry. The number 5, in this context, is not merely a numerical value but a key to unlocking a rich understanding of this intriguing metalloid.
Latest Posts
Latest Posts
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
-
The Standard Unit For Measuring Volume Is
Apr 02, 2025
-
Materials Like Rubber That Resist The Flow Of E
Apr 02, 2025
-
What Are The Units Of Conductance
Apr 02, 2025
-
What Is Mega In Scientific Notation
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Each Atom Of Arsenic Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
