Draw And Label A Ph Scale
News Leon
Apr 01, 2025 · 6 min read
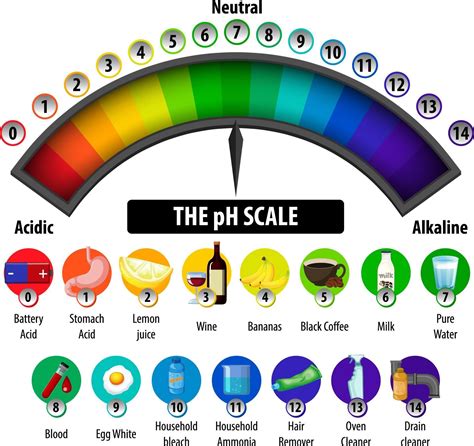
Table of Contents
Draw and Label a pH Scale: A Comprehensive Guide
The pH scale is a fundamental concept in chemistry and numerous other scientific disciplines. Understanding its workings is crucial for anyone studying chemistry, biology, environmental science, or even culinary arts. This comprehensive guide will not only explain how to draw and label a pH scale but also delve into its significance, applications, and the intricacies of its logarithmic nature.
Understanding the pH Scale
The pH scale measures the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 representing neutral. Solutions with a pH less than 7 are considered acidic, while solutions with a pH greater than 7 are alkaline or basic.
Key features of the pH scale:
-
Logarithmic Scale: The pH scale is logarithmic, meaning each whole number change represents a tenfold change in the concentration of hydrogen ions (H⁺). For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5.
-
Hydrogen Ion Concentration: The pH scale is directly related to the concentration of hydrogen ions (H⁺) in a solution. A higher concentration of H⁺ indicates a lower pH (more acidic), while a lower concentration of H⁺ indicates a higher pH (more alkaline).
-
Hydroxide Ion Concentration: While the pH scale focuses on hydrogen ions, it's interconnected with the concentration of hydroxide ions (OH⁻). In pure water, the concentrations of H⁺ and OH⁻ are equal, resulting in a neutral pH of 7. As acidity increases (H⁺ increases), the concentration of OH⁻ decreases, and vice-versa.
How to Draw and Label a pH Scale
Drawing a pH scale is straightforward. Here's a step-by-step guide:
-
Draw a Line: Draw a horizontal line of sufficient length.
-
Mark the Extremes: Mark the two ends of the line with "0" and "14". "0" represents the most acidic solution, and "14" represents the most alkaline solution.
-
Mark the Neutral Point: Mark the midpoint of the line with "7," representing a neutral solution.
-
Divide the Scale: Divide the line into equal intervals, typically representing whole numbers from 0 to 14. You can add more subdivisions for greater precision, showing tenths or hundredths.
-
Label the Intervals: Label each interval with its corresponding pH value.
-
Add Descriptive Labels (Optional): You can add descriptive labels to indicate the acidity or alkalinity of different regions of the scale. For example, you could label areas below 3 as "strongly acidic," 3-6 as "weakly acidic," 7 as "neutral," 8-11 as "weakly alkaline," and above 11 as "strongly alkaline."
-
Include Examples (Optional): Adding examples of substances with different pH values can enhance understanding. For instance, you could include:
- Strongly Acidic: Battery acid (pH ~ 1), stomach acid (pH ~ 2)
- Weakly Acidic: Lemon juice (pH ~ 2.4), vinegar (pH ~ 3)
- Neutral: Pure water (pH ~ 7)
- Weakly Alkaline: Baking soda solution (pH ~ 8), seawater (pH ~ 8.1)
- Strongly Alkaline: Household bleach (pH ~ 13), lye (pH ~ 14)
Visual Representation:
Imagine a horizontal line:
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Strongly Acidic Weakly Acidic Neutral Weakly Alkaline Strongly Alkaline
The Importance of the pH Scale
The pH scale has widespread applications across various fields:
1. Chemistry:
- Acid-Base Reactions: The pH scale is crucial in understanding and predicting the outcome of acid-base reactions. It helps determine whether a reaction will favor the formation of products or remain in equilibrium.
- Titrations: pH measurements are fundamental in titrations, a technique used to determine the concentration of an unknown solution using a solution of known concentration.
- Chemical Equilibrium: The pH influences chemical equilibrium by affecting the concentrations of various species in a solution.
2. Biology:
- Enzyme Activity: Enzymes, biological catalysts, function optimally within a specific pH range. Deviations from this range can lead to decreased or complete loss of enzyme activity.
- Cellular Processes: Many cellular processes are pH-dependent, including membrane transport, protein folding, and DNA replication.
- Blood pH: Maintaining a stable blood pH (around 7.4) is crucial for human health. Significant deviations can be life-threatening.
- Soil pH: Soil pH significantly impacts plant growth. Different plants thrive in different pH ranges.
3. Environmental Science:
- Water Quality: pH is a critical indicator of water quality. Changes in pH can affect aquatic life and ecosystem health. Acid rain, for example, lowers the pH of lakes and rivers, harming aquatic organisms.
- Pollution Monitoring: Monitoring pH levels in soil, water, and air helps track and assess the impact of various pollutants.
- Wastewater Treatment: pH control is essential in wastewater treatment processes to optimize the efficiency of various treatment steps.
4. Other Applications:
- Agriculture: Soil pH affects nutrient availability and plant growth. Farmers use pH measurements to optimize soil conditions for better crop yields.
- Food Industry: pH plays a vital role in food preservation, processing, and quality control. For instance, adjusting the pH can prevent bacterial growth and improve the shelf life of food products.
- Cosmetics and Personal Care: The pH of skincare and hair care products is crucial for maintaining the skin and hair's natural pH balance.
Measuring pH
Several methods are used to measure pH:
- pH Indicators: These are substances that change color depending on the pH of the solution. Litmus paper is a common example, turning red in acidic solutions and blue in alkaline solutions. More sophisticated pH indicators offer a wider range of color changes across different pH values.
- pH Meters: These electronic devices provide a more precise measurement of pH. They use a probe that measures the electrical potential difference between two electrodes, which is related to the hydrogen ion concentration. pH meters are widely used in laboratories and various industries.
Importance of Accurate pH Measurement
Accurate pH measurement is essential for numerous applications, as deviations can have significant consequences:
- Inaccurate measurements can lead to incorrect interpretations of experimental results, affecting research outcomes and decision-making.
- In industrial processes, incorrect pH can negatively impact product quality, efficiency, and safety.
- In environmental monitoring, inaccurate pH measurements can lead to misinterpretations of environmental conditions and potential harm to ecosystems.
- In healthcare, precise pH measurements are crucial for diagnosis and treatment of various medical conditions.
Conclusion
The pH scale is a fundamental tool with widespread implications across various scientific disciplines and industries. Understanding how to draw and label a pH scale, its logarithmic nature, and its applications is crucial for anyone seeking to grasp the chemical properties of solutions and their influence on biological and environmental systems. The accuracy of pH measurement is paramount, ensuring reliability in research, industrial processes, and environmental monitoring. Therefore, a comprehensive understanding of the pH scale is essential for effective scientific investigation, technological advancements, and environmental stewardship. Mastering this concept opens doors to a deeper understanding of the intricate world around us.
Latest Posts
Latest Posts
-
Distance Between Earth And Moon In Light Years
Apr 02, 2025
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
-
The Standard Unit For Measuring Volume Is
Apr 02, 2025
-
Materials Like Rubber That Resist The Flow Of E
Apr 02, 2025
-
What Are The Units Of Conductance
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Draw And Label A Ph Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
