In The Structure Of 4-isopropyl-2 4 5-trimethylheptane
News Leon
Mar 28, 2025 · 5 min read
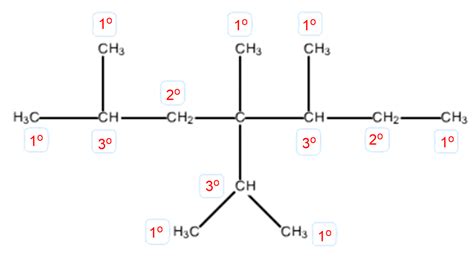
Table of Contents
Delving Deep into the Structure of 4-Isopropyl-2,4,5-Trimethylheptane
Understanding the structure of organic molecules is fundamental to chemistry. This article will provide a comprehensive exploration of 4-isopropyl-2,4,5-trimethylheptane, a complex branched alkane, covering its systematic nomenclature, structural isomerism, conformational analysis, physical properties, and potential applications. We will use various tools and techniques to visualize and analyze its three-dimensional structure.
Understanding the IUPAC Nomenclature
The name "4-isopropyl-2,4,5-trimethylheptane" itself reveals much about the molecule's structure. Let's break it down systematically using IUPAC (International Union of Pure and Applied Chemistry) rules:
- Heptane: This indicates the parent chain contains seven carbon atoms.
- 2,4,5-trimethyl: This signifies three methyl groups (CH₃) attached to carbon atoms 2, 4, and 5 of the heptane chain.
- 4-isopropyl: This denotes an isopropyl group [(CH₃)₂CH] attached to carbon atom 4.
This nomenclature provides a blueprint for constructing the molecule's skeletal structure.
Constructing the Skeletal Structure
-
Draw the heptane chain: Begin by drawing a straight chain of seven carbon atoms.
-
Add the methyl groups: Attach methyl groups to carbons 2, 4, and 5.
-
Add the isopropyl group: Attach the isopropyl group to carbon 4.
It's crucial to note that the numbering of the carbon atoms is determined to provide the lowest possible numbers for the substituents. Different numbering schemes would lead to a different, but equally valid, name, although less preferable according to IUPAC conventions. This emphasizes the importance of a systematic approach to naming.
Visualizing the 3D Structure
While the 2D representation gives a good understanding of connectivity, it doesn't fully capture the three-dimensional arrangement of atoms. A true understanding necessitates visualizing the molecule in 3D. Various methods can achieve this:
-
Molecular Modeling Software: Programs like ChemDraw, Avogadro, or Spartan provide powerful tools for building and visualizing 3D molecular models. These tools allow for rotation and manipulation of the molecule, providing a better grasp of its spatial arrangement.
-
Ball-and-stick models: A classic method involves using physical ball-and-stick models. While potentially time-consuming, this hands-on approach reinforces understanding of molecular geometry.
-
Space-filling models: These models depict the molecule as a collection of spheres representing atoms, accurately reflecting the relative sizes of atoms. This helps visualize steric effects, which are crucial in understanding the molecule's properties and reactivity.
Conformational Analysis
4-isopropyl-2,4,5-trimethylheptane possesses numerous conformations due to the free rotation around its single carbon-carbon bonds. These conformations differ in energy, resulting in some being more stable than others. Factors influencing conformational stability include:
-
Steric hindrance: Bulky groups, like the isopropyl and methyl groups, will experience steric repulsion when positioned closely. Conformers with minimal steric interactions are generally more stable.
-
Gauche and anti interactions: Gauche interactions (groups at a 60° dihedral angle) are less stable than anti interactions (groups at a 180° dihedral angle) due to steric effects.
Detailed conformational analysis would involve energy calculations using computational chemistry methods (like MMFF94 or DFT). This analysis would identify the lowest-energy conformers and their relative populations at different temperatures. This information is critical for predicting the molecule's physical and chemical properties.
Stereoisomerism
While 4-isopropyl-2,4,5-trimethylheptane does not exhibit optical isomerism (chirality), it is crucial to consider the possibility of other forms of isomerism:
-
Constitutional Isomerism: This refers to molecules with the same molecular formula but different connectivity. Numerous constitutional isomers exist with the same formula as 4-isopropyl-2,4,5-trimethylheptane. Identifying these isomers would require systematic exploration of all possible arrangements of the carbon atoms and substituents.
-
Geometric Isomerism: This type of isomerism is not applicable to alkanes since it requires the presence of double bonds or rings.
Physical Properties
The physical properties of 4-isopropyl-2,4,5-trimethylheptane are significantly influenced by its structure:
-
Boiling point: The molecule's branched structure leads to a lower boiling point compared to its linear isomer, n-nonane. This is because branched molecules have weaker London dispersion forces due to reduced surface area contact.
-
Melting point: Similar to the boiling point, the melting point will also be affected by the branched structure. Predicting the precise melting point requires experimental determination or advanced computational methods.
-
Density: The density will be less than 1 g/mL, typical for alkanes.
-
Solubility: As a non-polar molecule, it is insoluble in water but soluble in non-polar organic solvents.
-
Viscosity: It will have a relatively low viscosity compared to longer, unbranched alkanes.
Determining the precise values of these properties would necessitate either experimental measurement or sophisticated computational chemistry calculations.
Potential Applications
While not a commonly used compound with readily available commercial applications, understanding the properties of 4-isopropyl-2,4,5-trimethylheptane can have implications in various fields:
-
Organic synthesis: It could serve as a starting material or intermediate in the synthesis of more complex molecules. Its branched structure can influence the regioselectivity and stereoselectivity of reactions.
-
Fuel research: As an alkane, it could be studied as a component in fuel blends. The molecule's combustion characteristics are relevant in understanding the properties of complex fuel mixtures.
-
Solvent studies: Its non-polar nature makes it a potential candidate as a solvent for non-polar compounds. However, its potential use would be limited due to the presence of more readily available solvents with comparable properties.
Conclusion
4-isopropyl-2,4,5-trimethylheptane, despite its seemingly complex name, is a fascinating molecule that allows for a thorough exploration of various concepts in organic chemistry. Understanding its nomenclature, 3D structure, conformational analysis, and predicted physical properties provides a solid foundation for comprehending the behavior and potential applications of complex branched alkanes. Although its direct applications might be limited, its study offers valuable insights into broader organic chemistry principles. Further investigation, using both experimental and computational methods, would be necessary to fully elucidate its physical and chemical properties. The ability to accurately predict these properties would prove invaluable in various scientific and engineering endeavors.
Latest Posts
Latest Posts
-
Which Of The Following Are Complementary Bases In Dna
Mar 31, 2025
-
Classify The Following As A Homogeneous Or A Heterogeneous Mixture
Mar 31, 2025
-
What Physical Quantity Does The Slope Represent
Mar 31, 2025
-
An Object Becomes Positively Charged By Gaining Protons
Mar 31, 2025
-
Right Hand Rule For Angular Velocity
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about In The Structure Of 4-isopropyl-2 4 5-trimethylheptane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
