In A Hypertonic Solution A Cell Will
News Leon
Mar 26, 2025 · 6 min read
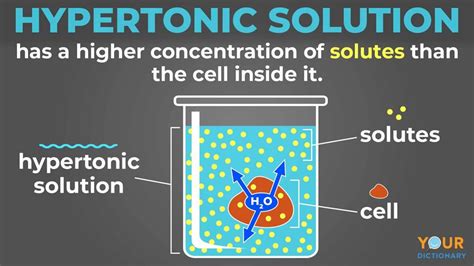
Table of Contents
In a Hypertonic Solution, a Cell Will… Shrivel Up! Understanding Osmosis and its Effects
Understanding how cells behave in different environments is fundamental to biology. One crucial concept is osmosis, the movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement is driven by the difference in water potential, aiming to achieve equilibrium. When a cell is placed in a hypertonic solution, a dramatic change occurs, affecting its structure and function. This article delves deep into the process, exploring the mechanics of osmosis in a hypertonic environment, its effects on various cell types, and the broader implications for biological systems.
What is a Hypertonic Solution?
A hypertonic solution is one in which the concentration of solutes (dissolved substances) is higher outside the cell than inside the cell. This means the water concentration is lower outside the cell compared to inside. Think of it like this: imagine a crowded room (high solute concentration) versus an empty room (low solute concentration). Water naturally wants to move from the empty room (inside the cell) to the crowded room (outside the cell) to dilute the crowded space. This crucial difference in solute concentration drives the osmotic process.
Key Components of a Hypertonic Environment:
- High Solute Concentration: The external environment contains a significantly higher concentration of dissolved substances like salts, sugars, or other molecules.
- Low Water Potential: The water potential outside the cell is lower than inside the cell, creating a water concentration gradient.
- Selectively Permeable Membrane: The cell membrane acts as a barrier, allowing water to pass through but restricting the movement of many solutes.
The Osmosis Process in a Hypertonic Environment: Water Exodus
When a cell is immersed in a hypertonic solution, water molecules move out of the cell across the selectively permeable membrane. This movement is a passive process, meaning it doesn't require energy input from the cell. The driving force is the difference in water potential – water moves to equalize the concentration gradient.
Step-by-step breakdown:
- Concentration Gradient: A difference in water concentration exists between the cell's interior and the surrounding hypertonic solution.
- Water Movement: Water molecules, due to their random thermal motion, constantly move across the membrane. However, the net movement is from the high water concentration (inside the cell) to the low water concentration (outside the cell).
- Osmotic Pressure: The pressure exerted by the water moving across the membrane is known as osmotic pressure. In a hypertonic solution, this pressure pushes water out of the cell.
- Cell Shrinkage: As water leaves the cell, the cell's cytoplasm shrinks, and the cell membrane pulls away from the cell wall (in plant cells) or becomes crenated (in animal cells).
Effects on Different Cell Types: Plant vs. Animal Cells
The effects of a hypertonic solution differ between plant and animal cells due to the presence of a rigid cell wall in plant cells.
Plant Cells in Hypertonic Solutions: Plasmolysis
In plant cells, the cell wall provides structural support. When placed in a hypertonic solution, water flows out of the cell, causing the cell membrane to detach from the cell wall. This process is called plasmolysis. The cell becomes flaccid, losing its turgor pressure, and may eventually wilt. Plasmolysis is a reversible process; if the plant cell is returned to a hypotonic or isotonic solution, it can regain its turgor pressure.
Animal Cells in Hypertonic Solutions: Crenation
Animal cells lack a rigid cell wall. When placed in a hypertonic solution, water rushes out of the cell, causing it to shrink and become wrinkled. This process is called crenation. Crenation can significantly disrupt cell function and may ultimately lead to cell death if the water loss is substantial.
The Significance of Hypertonic Environments in Biology
The principles of osmosis and the effects of hypertonic solutions are vital in numerous biological processes and have practical implications:
1. Water Regulation in Organisms:
Maintaining water balance is crucial for all living organisms. Hypertonic environments pose a challenge, as cells need mechanisms to prevent excessive water loss. Specialized adaptations, such as contractile vacuoles in certain protists, help to expel excess water and maintain osmotic balance.
2. Food Preservation:
Hypertonic solutions are often used in food preservation techniques like pickling and salting. The high solute concentration in these solutions draws water out of microorganisms, inhibiting their growth and preventing spoilage. The process effectively dehydrates the microbes, rendering them unable to multiply and potentially harmful.
3. Medical Applications:
Understanding osmosis is critical in medicine. Intravenous (IV) fluids must have a specific tonicity to prevent damage to red blood cells. Using a hypertonic solution intravenously would cause the red blood cells to crenate, potentially leading to serious complications. The careful selection of isotonic solutions ensures that red blood cells maintain their normal shape and function.
4. Plant Physiology:
In plant biology, understanding plasmolysis helps explain wilting and the importance of maintaining adequate water availability for optimal growth and function. Farmers and gardeners use this knowledge to manage irrigation effectively to prevent water stress in crops and prevent wilting.
5. Cellular Transport:
Osmosis is intrinsically linked to other transport mechanisms across cell membranes. The movement of water influences the concentration gradients of other substances, affecting their transport rates and impacting cellular processes. The maintenance of an appropriate osmotic balance, therefore, is vital for overall cellular function.
Beyond the Basics: Factors Affecting Osmosis in Hypertonic Environments
Several factors can influence the rate and extent of osmosis in a hypertonic solution:
- Solute Concentration Gradient: The steeper the concentration gradient (the greater the difference in solute concentration between inside and outside the cell), the faster the rate of water movement.
- Membrane Permeability: The permeability of the cell membrane to water and other solutes affects the rate of osmosis. Membranes with higher water permeability will experience faster water movement.
- Temperature: Higher temperatures generally increase the rate of molecular movement, including water molecules, thereby accelerating osmosis.
- Surface Area: A larger surface area of the cell membrane exposed to the hypertonic solution allows for a faster rate of water movement.
- Type of Solute: Different solutes have different effects on water potential, even at the same concentration. The type of solute in the hypertonic solution can influence the overall osmotic effect on the cell.
Conclusion: A Delicate Balance
The behavior of cells in a hypertonic solution highlights the critical role of osmosis in maintaining cellular homeostasis. The movement of water, driven by the concentration gradient, leads to significant changes in cell structure and function. Understanding these processes is fundamental to comprehending various biological phenomena, from water regulation in organisms to the preservation of food and the development of medical treatments. The delicate balance of water and solutes within and around cells is essential for life, and the impact of a hypertonic environment emphasizes the importance of maintaining this equilibrium. The study of osmosis in hypertonic solutions continues to reveal crucial insights into the intricate workings of living systems. Further research will undoubtedly uncover more nuanced details of this fundamental biological process and its far-reaching consequences.
Latest Posts
Latest Posts
-
How Much Seconds Are In A Week
Mar 29, 2025
-
Is Boron A Gas Solid Or Liquid
Mar 29, 2025
-
How Do You Factor 2x 2 7x 3
Mar 29, 2025
-
What Is 0 025 As A Percentage
Mar 29, 2025
-
Integrated Rate Law For Zero Order Reaction
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about In A Hypertonic Solution A Cell Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
