Is Boron A Gas Solid Or Liquid
News Leon
Mar 29, 2025 · 5 min read
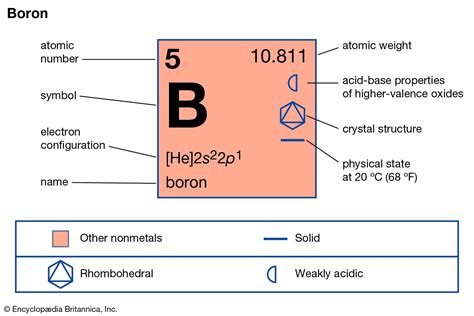
Table of Contents
Is Boron a Gas, Solid, or Liquid? Understanding Boron's Unique Properties
Boron, a metalloid element with the symbol B and atomic number 5, presents a fascinating case study in the states of matter. Unlike many elements that exist predominantly in one state at standard temperature and pressure (STP), boron's story is more nuanced. This article delves deep into the nature of boron, exploring its unique properties and explaining why classifying it as simply a gas, solid, or liquid is an oversimplification.
Boron: A Metalloid with a Crystalline Structure
At STP, boron exists as a solid. This is a crucial point to establish before moving into more complex discussions. It's not a gas like oxygen or a liquid like mercury. Its solid state is characterized by a crystalline structure, meaning its atoms are arranged in a highly ordered, repeating pattern. This crystalline structure is a key factor influencing its properties.
The Complexity of Boron's Crystalline Forms
The statement that boron is a solid needs some qualification. While it's undeniably solid at room temperature, boron doesn't form a single, simple crystal structure like many other elements. Instead, it exhibits multiple allotropes, meaning it exists in different structural forms. These forms differ in their arrangement of boron atoms and, consequently, in their physical and chemical properties.
- α-rhombohedral boron: This is the most common allotrope and is characterized by a complex structure involving 12 icosahedral units (clusters of 12 boron atoms arranged in a geometric shape).
- β-rhombohedral boron: This allotrope features a more complex structure than the alpha form, containing 105 boron atoms.
- γ-rhombohedral boron: Yet another allotrope, showcasing the structural complexity that boron displays.
- Tetragonal boron: This less common allotrope has a different structural arrangement compared to the rhombohedral forms.
The differences in these allotropes impact the overall properties of boron, such as its hardness, density, and reactivity. This complexity makes it essential to avoid a simplistic answer when discussing the state of boron. It's not simply a "solid"; it's a solid with multiple crystalline expressions.
Why Boron Doesn't Exist as a Gas or Liquid at Standard Conditions
The reason boron is a solid at STP relates directly to its atomic structure and bonding. Boron atoms are relatively small and have a strong tendency to form covalent bonds with each other. These strong covalent bonds create a robust three-dimensional network within the crystalline structure. This network requires a significant amount of energy to break apart, making it extremely difficult for boron to transition to a liquid or gaseous state under normal conditions.
High Melting and Boiling Points: A Consequence of Strong Bonding
The strength of these covalent bonds is directly reflected in boron's exceptionally high melting point (around 2076 °C or 2349 K) and boiling point (around 3927 °C or 4200 K). These incredibly high values demonstrate the substantial amount of energy needed to overcome the strong interatomic forces holding boron's solid structure together. Therefore, to see boron as a liquid or gas would necessitate extremely high temperatures far beyond those experienced under ordinary circumstances.
Comparing Boron to Other Elements: The Metalloid Nature
Boron's behavior contrasts sharply with many other elements. Gases, such as oxygen and nitrogen, have weak intermolecular forces, allowing them to exist as individual molecules and readily transition to the gaseous phase at relatively low temperatures. Liquids, like water and mercury, have stronger intermolecular forces than gases, but still relatively weaker than the bonding found in boron. This makes them more likely to transition states. Boron, being a metalloid, sits between metals and nonmetals, exhibiting properties of both. Its strong covalent bonding puts it firmly in the realm of solids under normal conditions.
Exploring Boron's Behavior Under Extreme Conditions
While boron is predominantly solid at STP, its behavior can change under extreme conditions. At extremely high temperatures, well beyond its boiling point, boron could theoretically exist as a gas. Similarly, at very high pressures, it might exhibit different crystalline structures, or perhaps even amorphous forms (lacking a well-defined crystalline structure). These scenarios, however, are far removed from everyday experiences.
Amorphous Boron: A Non-Crystalline Form
It's important to note the existence of amorphous boron. This form lacks the ordered crystalline structure characteristic of the other allotropes. It's produced through methods that don't allow sufficient time for crystallization during the boron's formation. Even in this amorphous form, however, boron remains a solid at standard temperatures. The absence of a crystal lattice doesn't change the fact that the element remains a solid.
The Importance of Precision in Scientific Terminology
The discussion surrounding boron's state underscores the importance of precise scientific language. While it's tempting to simply label boron as a "solid," a more thorough understanding reveals the complexity of its crystalline forms and its behavior under different conditions. This complexity is typical of many materials, highlighting the value of deeper investigation beyond simplistic classifications.
Beyond the Basics: Applications and Significance
Boron, despite its unusual properties, plays a crucial role in various applications. It is used in the production of:
- High-strength materials: Boron fibers are used to reinforce composites, leading to lightweight yet incredibly strong materials used in aerospace and other high-performance applications.
- Semiconductors: Boron is a crucial dopant in semiconductors, modifying their electrical properties to create electronic devices.
- Nuclear applications: Boron's ability to absorb neutrons is exploited in nuclear reactors for control rod applications.
- Detergents and cleaning products: Boron compounds are used in some detergents and cleaning agents.
These applications highlight the crucial importance of understanding boron's unique characteristics – its solid nature at STP, its various crystalline forms, and its extraordinary high melting and boiling points.
Conclusion: A Solid, but Complex Element
In summary, boron is a solid at standard temperature and pressure. Its solid state is defined by its various crystalline allotropes, each with distinct structural arrangements. The exceptionally strong covalent bonds between boron atoms account for its high melting and boiling points, making it highly unlikely to observe boron as a gas or liquid except under exceptionally extreme conditions. The complex nature of boron, both in its physical states and its wide-ranging applications, underscores the importance of detailed scientific understanding beyond simple classifications. It is a solid, but a solid deserving of a more thorough and nuanced description than simply stating its state of matter at STP.
Latest Posts
Latest Posts
-
Which Of The Following Best Describes Climate
Apr 01, 2025
-
What Are The Parts Of A Solution
Apr 01, 2025
-
What Is The Value Of 3 4
Apr 01, 2025
-
Cation And Anion Held Together By Electrostatic Forces
Apr 01, 2025
-
Why Is Fluorine More Electronegative Than Chlorine
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Boron A Gas Solid Or Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
