How To Separate Sugar And Water
News Leon
Mar 21, 2025 · 6 min read
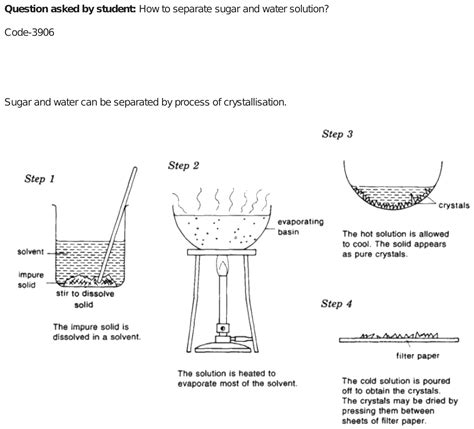
Table of Contents
How to Separate Sugar and Water: A Comprehensive Guide
Separating sugar and water might seem like a simple task, but understanding the underlying principles and exploring different methods can be surprisingly insightful. This comprehensive guide delves into the science behind sugar-water solutions and provides detailed explanations of various separation techniques, suitable for different scales and scenarios. Whether you're a curious student, a passionate home chef, or a budding scientist, this article will equip you with the knowledge to effectively separate sugar and water.
Understanding Sugar and Water: A Molecular Perspective
Before diving into separation methods, it's crucial to understand the nature of a sugar-water solution. When sugar (sucrose) is dissolved in water, it undergoes a process called dissolution. The sugar molecules, initially held together by strong intermolecular forces in the crystalline structure, interact with the polar water molecules. The slightly positive hydrogen atoms of water molecules attract the negatively charged oxygen atoms in the sugar molecules, and vice versa. This interaction weakens the bonds within the sugar crystal, causing the sugar molecules to break away and become dispersed throughout the water. The result is a homogeneous mixture where sugar molecules are evenly distributed among water molecules. Crucially, no chemical change occurs; the sugar and water retain their individual chemical identities. This is a key factor in determining the most effective separation techniques.
Methods for Separating Sugar and Water
Several methods can be employed to separate sugar from water, each with its own advantages and disadvantages. The optimal choice depends on factors like the quantity of the solution, the desired purity of the separated components, and the available resources.
1. Evaporation
This is perhaps the simplest and most commonly understood method. Evaporation relies on the difference in boiling points between water (100°C at standard atmospheric pressure) and sugar (which decomposes before reaching its boiling point).
The Process:
- Heat the sugar-water solution: Gently heat the solution in a suitable container, such as a saucepan or beaker. A slow and even heat distribution is crucial to prevent bumping and splattering.
- Water evaporates: As the solution heats, the water molecules gain kinetic energy and transition from the liquid phase to the gaseous phase, escaping into the atmosphere.
- Sugar remains: The sugar, with its much higher boiling point, remains behind in the container as a concentrated syrup, and eventually as dry crystals.
Advantages:
- Simplicity: This is a straightforward method requiring minimal equipment.
- Effectiveness: It effectively separates the water, leaving behind relatively pure sugar.
Disadvantages:
- Time-consuming: Evaporation can be a slow process, especially for large volumes of solution.
- Energy intensive: It requires sustained heating, consuming significant energy.
- Sugar caramelization: If the temperature is too high, the sugar can caramelize, altering its chemical properties and potentially creating a burnt or undesired flavor.
2. Distillation
Distillation is a more sophisticated method that is particularly useful for separating liquids with different boiling points. While evaporation simply allows the water to escape, distillation actively collects the vaporized water.
The Process:
- Heating and Vaporization: The sugar-water solution is heated in a distillation flask. The water evaporates, forming steam.
- Condensation: The steam travels through a condenser, a tube cooled by running water. This cools the steam, causing it to condense back into liquid water.
- Collection: The condensed water (distillate) is collected in a receiving flask, leaving the sugar behind in the distillation flask.
Advantages:
- High Purity: Distillation yields purer water compared to simple evaporation.
- Efficiency: For larger volumes, distillation can be more efficient than simple evaporation.
Disadvantages:
- Complex Setup: Distillation requires specialized equipment like a distillation apparatus, condenser, and heat source.
- Cost: The equipment involved can be expensive.
3. Chromatography
Chromatography is a powerful technique used to separate components of a mixture based on their differing affinities for a stationary and mobile phase. While less commonly used for sugar and water separation at a household level, it's a valuable technique in various scientific applications.
The Process:
Different types of chromatography exist, but a common example involves using paper chromatography. A small amount of the sugar-water solution is placed on a piece of filter paper. The paper is then dipped into a solvent (the mobile phase). The solvent moves up the paper, carrying the water and sugar with it, but at different rates due to their different interactions with the paper (stationary phase). This allows for separation of the components.
Advantages:
- Precise Separation: It's highly effective for separating complex mixtures.
- Small Sample Size: It can be used with very small amounts of solution.
Disadvantages:
- Technical Expertise: Requires specialized knowledge and equipment.
- Not Ideal for Large Scales: Not practical for separating large quantities of sugar and water.
4. Reverse Osmosis
Reverse osmosis is a membrane filtration process that uses pressure to force water through a semipermeable membrane. The sugar molecules, being larger than the water molecules, are unable to pass through the membrane.
The Process:
The sugar-water solution is pressurized and forced against a semipermeable membrane. The water molecules pass through the membrane, leaving the sugar molecules behind.
Advantages:
- High Efficiency: Can effectively separate even dilute solutions.
- Versatile: Applicable to various types of solutions.
Disadvantages:
- Specialized Equipment: Requires expensive and specialized equipment, including a high-pressure pump and specialized membranes.
- Maintenance: Membranes can clog and require regular cleaning or replacement.
Choosing the Right Method
The best method for separating sugar and water depends on your specific needs and resources.
- For small quantities and simplicity, evaporation is the easiest option.
- For larger quantities and higher purity water, distillation is preferable.
- Chromatography is best suited for analytical purposes and separating complex mixtures.
- Reverse osmosis is a more advanced technique for efficient separation, particularly in industrial applications.
Safety Precautions
Regardless of the chosen method, safety should always be a priority:
- Heat-related safety: When using heat, always exercise caution to avoid burns. Use heat-resistant gloves and protective eyewear.
- Glassware: Handle glassware carefully to prevent breakage.
- Electrical safety: If using electrical equipment, ensure it's properly grounded and in good working order.
Conclusion
Separating sugar and water involves leveraging the different physical properties of these two substances. Understanding the science behind the process and the various available techniques empowers you to choose the most effective and safe method for your specific situation. From the simple technique of evaporation to the more complex process of reverse osmosis, the choice depends on factors such as scale, purity requirements, and available resources. Remember to always prioritize safety and follow appropriate procedures when performing these experiments. This detailed explanation provides a foundation for further exploration and understanding of separation techniques in chemistry and related fields.
Latest Posts
Latest Posts
-
Choose The Correct Answer From The Given Options
Mar 27, 2025
-
In The Figure A Metal Rod Is Forced To Move
Mar 27, 2025
-
Do Covalent Compounds Dissolve In Water
Mar 27, 2025
-
What Is The Specific Heat Capacity Of Aluminium
Mar 27, 2025
-
Do Noble Gases Have Ionization Energy
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How To Separate Sugar And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
