Do Covalent Compounds Dissolve In Water
News Leon
Mar 27, 2025 · 6 min read
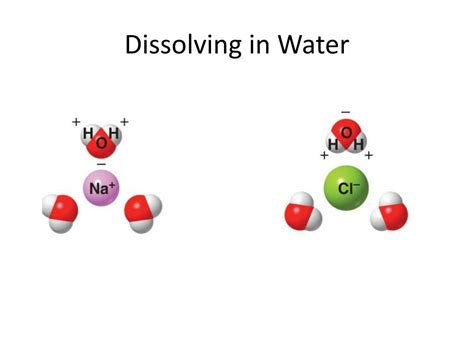
Table of Contents
Do Covalent Compounds Dissolve in Water? A Deep Dive into Solubility
The question of whether covalent compounds dissolve in water is a complex one, not answered by a simple yes or no. While the general rule of thumb suggests that ionic compounds dissolve in water while covalent compounds do not, the reality is far more nuanced. The solubility of a covalent compound in water depends on several factors, making it a fascinating topic to explore. This article will delve into the intricacies of covalent compound solubility, examining the various forces at play and providing a deeper understanding of this fundamental chemical concept.
Understanding the Nature of Covalent Bonds and Water Molecules
Before diving into the solubility of covalent compounds, let's establish a foundational understanding of the key players involved: covalent bonds and water molecules.
Covalent Bonds: Sharing is Caring
A covalent bond is formed when two atoms share one or more pairs of electrons. This sharing results in a relatively strong bond, holding the atoms together to form molecules. Examples of covalent compounds include methane (CH₄), glucose (C₆H₁₂O₆), and ethanol (C₂H₅OH). The strength of these bonds varies depending on the atoms involved and the number of shared electron pairs.
Water: The Universal Solvent (But Not for Everything)
Water (H₂O) is a polar molecule. This means it has a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity arises from the difference in electronegativity between oxygen and hydrogen. Oxygen is more electronegative, attracting the shared electrons more strongly and creating a partial negative charge. This polarity is crucial to water's ability to dissolve many substances.
Factors Affecting the Solubility of Covalent Compounds in Water
The solubility of a covalent compound in water hinges on several key factors:
1. Polarity: The Key Player
The most significant factor influencing the solubility of a covalent compound in water is its polarity. Polar covalent compounds, possessing a significant difference in electronegativity between atoms, often dissolve in water because their polar molecules can interact with water molecules through dipole-dipole interactions. These interactions are electrostatic attractions between the positive end of one polar molecule and the negative end of another. The slightly positive hydrogen atoms in water are attracted to the slightly negative atoms in the polar covalent compound, while the slightly negative oxygen atom in water is attracted to the slightly positive atoms in the covalent compound. This interaction effectively surrounds the covalent compound molecules, allowing them to dissolve.
Examples of polar covalent compounds that dissolve in water: Ethanol (C₂H₅OH), glucose (C₆H₁₂O₆), and ammonia (NH₃).
2. Hydrogen Bonding: A Special Case of Dipole-Dipole Interaction
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine). This creates a strong partial positive charge on the hydrogen atom, which can strongly attract the lone pairs of electrons on the oxygen atom in a water molecule. Compounds capable of forming hydrogen bonds with water tend to be highly soluble.
Examples of covalent compounds with strong hydrogen bonding that dissolve well in water: Glucose (numerous hydroxyl groups capable of hydrogen bonding), and acetic acid (CH₃COOH).
3. Size and Shape of the Molecule: Steric Effects
The size and shape of the covalent molecule also influence solubility. Larger molecules often have lower solubility because more water molecules are required to surround and solvate them. The shape of the molecule also plays a role. A compact, spherical shape facilitates easier solvation compared to a long, irregularly shaped molecule.
4. Presence of Nonpolar Regions: Impact of Hydrophobic Interactions
Covalent compounds that possess both polar and nonpolar regions exhibit amphipathic behavior. These molecules have a polar (hydrophilic – water-loving) portion that interacts favorably with water and a nonpolar (hydrophobic – water-fearing) portion that repels water. The balance between hydrophilic and hydrophobic interactions determines the overall solubility. Molecules with large nonpolar regions are often less soluble in water.
Example: Fatty acids have a polar carboxyl group (-COOH) and a long, nonpolar hydrocarbon chain. The solubility of fatty acids decreases as the length of the hydrocarbon chain increases.
5. Temperature: A Temperature-Dependent Relationship
Temperature can significantly influence the solubility of covalent compounds in water. Generally, increasing the temperature increases the kinetic energy of molecules, enhancing interactions between water and the solute, thus boosting solubility. However, there are exceptions, and the relationship between temperature and solubility is specific to each compound.
Covalent Compounds That Don't Dissolve in Water: The Insoluble Side
Many covalent compounds are insoluble in water. These compounds are typically nonpolar or have a very small polar region compared to their overall size. The lack of strong attractive forces between the covalent compound molecules and water molecules prevents significant dissolution.
Examples of largely insoluble covalent compounds: Most hydrocarbons (e.g., hexane, octane), oils, and fats.
The Role of Intermolecular Forces in Solubility
Understanding the various types of intermolecular forces is crucial for predicting the solubility of covalent compounds in water. The strength of these forces dictates how effectively a covalent compound can interact with water molecules.
1. Dipole-Dipole Interactions: Polarity Matters
As previously mentioned, dipole-dipole interactions occur between polar molecules. The strength of these interactions influences solubility. Stronger dipole-dipole interactions lead to higher solubility.
2. London Dispersion Forces: Weak but Present
Even nonpolar molecules experience weak attractive forces called London dispersion forces (LDFs). These forces arise from temporary fluctuations in electron distribution, creating temporary dipoles. While these forces are weak, they can still contribute to the overall interaction between the solute and water molecules, though usually to a lesser extent than dipole-dipole forces or hydrogen bonding.
3. Ion-Dipole Interactions: When Ions Are Involved
Some covalent compounds can partially ionize in water, creating ions that interact with water molecules through ion-dipole interactions. This interaction is stronger than dipole-dipole interactions and can significantly increase solubility. For example, carboxylic acids like acetic acid partially ionize in water, increasing their solubility.
Conclusion: A Spectrum of Solubility
The solubility of covalent compounds in water is not a black-and-white issue. It's a complex interplay of factors including polarity, hydrogen bonding, molecular size and shape, the presence of hydrophobic regions, temperature, and the types of intermolecular forces involved. While some covalent compounds readily dissolve in water due to their strong polar nature and ability to form hydrogen bonds, many others remain largely insoluble due to the predominance of nonpolar regions or weak intermolecular interactions with water. Understanding these factors is critical for predicting and explaining the solubility behavior of covalent compounds and for countless applications in chemistry and related fields. This nuanced understanding extends beyond basic solubility rules, allowing for a deeper appreciation of the complex interactions that govern the behavior of matter at a molecular level. Further research into specific compounds and their unique molecular structures is necessary for precise solubility predictions.
Latest Posts
Latest Posts
-
The Only Movable Joint In The Skull Is Between The
Mar 31, 2025
-
Which Inequality Is Shown In The Graph Below
Mar 31, 2025
-
Which Mrna Sequence Complements The Dna Sequence Below
Mar 31, 2025
-
Square Root Of 6 Is Irrational
Mar 31, 2025
-
A Record Is A Collection Of Related Tables
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Do Covalent Compounds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
