Do Noble Gases Have Ionization Energy
News Leon
Mar 27, 2025 · 5 min read
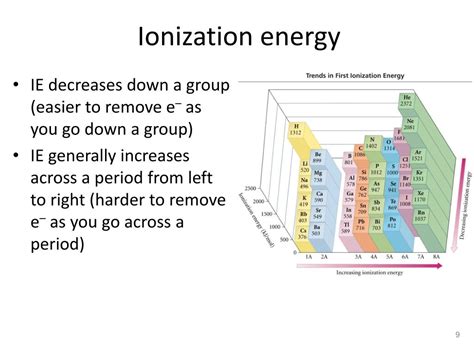
Table of Contents
Do Noble Gases Have Ionization Energy? Exploring the Ionization Energies of Inert Elements
Noble gases, also known as inert gases, are renowned for their chemical inactivity. This inertness stems from their unique electronic configurations, possessing a full valence electron shell. But does this complete shell mean they lack ionization energy altogether? The answer, surprisingly, is no. While they exhibit exceptionally high ionization energies, signifying their reluctance to lose electrons, they do possess ionization energy. This article delves deep into the concept of ionization energy in noble gases, exploring the factors influencing their high values, the trends observed across the noble gas group, and the exceptions that prove the rule.
Understanding Ionization Energy
Before focusing on noble gases specifically, let's establish a fundamental understanding of ionization energy. Ionization energy (IE) is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. This process results in the formation of a positively charged ion (cation). The first ionization energy (IE₁) refers to the removal of the first electron, the second ionization energy (IE₂) refers to the removal of the second electron, and so on. Generally, successive ionization energies increase, as removing each subsequent electron becomes progressively more difficult due to the increasing positive charge of the ion.
The magnitude of ionization energy is directly influenced by several factors:
- Nuclear Charge: A higher nuclear charge exerts a stronger attractive force on the electrons, leading to a higher ionization energy.
- Atomic Radius: A smaller atomic radius means the electrons are closer to the nucleus, experiencing a stronger attractive force and consequently a higher ionization energy.
- Shielding Effect: Inner electrons shield outer electrons from the full effect of the nuclear charge. A greater shielding effect reduces the effective nuclear charge experienced by the outer electrons, resulting in a lower ionization energy.
- Electron-Electron Repulsion: Repulsion between electrons within the same shell can counteract the attractive force of the nucleus, slightly lowering ionization energy.
The Exceptionally High Ionization Energies of Noble Gases
Noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon) occupy Group 18 of the periodic table. Their characteristic feature is their complete valence electron shell. This full octet (or duet in the case of Helium) provides exceptional stability, making them extremely reluctant to lose electrons. This inherent stability directly translates to extraordinarily high ionization energies.
Helium (He): The Pioneer of Inertness
Helium, with its electron configuration of 1s², boasts the highest first ionization energy of all elements. Its small atomic radius and strong nuclear charge, combined with the absence of any shielding effect, contribute significantly to its high IE₁. Removing an electron from helium requires a substantial amount of energy to overcome the strong attractive force of the nucleus.
Neon (Ne) and Argon (Ar): The Trend Continues
Moving down the group, Neon (Ne, [He]2s²2p⁶) and Argon (Ar, [Ne]3s²3p⁶) exhibit even higher ionization energies than Helium, although the increase isn't linear. While the nuclear charge increases, so does the shielding effect. The increase in atomic size slightly offsets the increase in nuclear charge. However, the strong attractive force on the valence electrons, owing to the compact arrangement, still results in exceptionally high ionization energies.
Krypton (Kr), Xenon (Xe), and Radon (Rn): The Gradual Decrease
As we progress to Krypton (Kr), Xenon (Xe), and Radon (Rn), the ionization energies gradually decrease. This is primarily due to the increasing atomic size. The larger atomic radius means the valence electrons are farther from the nucleus, experiencing a weaker attractive force. The shielding effect by inner electrons also plays a significant role, further reducing the effective nuclear charge experienced by the outermost electrons. Therefore, despite the increasing nuclear charge, the increased atomic size and shielding effect outweigh the nuclear charge increase, leading to a decrease in ionization energy.
Factors Influencing Ionization Energy Trends in Noble Gases
The observed trend of gradually decreasing ionization energies down the noble gas group is a complex interplay of several factors:
- Increasing Atomic Radius: The dominant factor contributing to the decrease is the increasing atomic size. Larger atoms have their valence electrons farther away from the nucleus, making them easier to remove.
- Increased Shielding Effect: The number of inner electron shells increases down the group, leading to a greater shielding effect. This reduces the effective nuclear charge experienced by the valence electrons.
- Relativistic Effects: In heavier noble gases like Radon, relativistic effects play a subtle but significant role. Relativistic effects involve the increased mass of inner electrons at high speeds, leading to slight contractions in the electron orbitals. This contraction can slightly increase the ionization energy, partially counteracting the trend of decreasing ionization energy down the group.
Exceptions and Anomalies: The Case of Helium
While the general trend of decreasing ionization energies down the noble gas group holds, Helium presents a notable exception. Its extremely high ionization energy is a unique outlier, reflecting its exceptionally small atomic radius and absence of any shielding effect. The strong nuclear charge, exerted directly on its two electrons, requires a significantly larger amount of energy to remove an electron compared to the other noble gases.
Applications and Significance
The extraordinarily high ionization energies of noble gases have significant implications in various fields:
- Lasers: Noble gases are used in lasers due to their ability to emit light when excited. Their high ionization energies make them resistant to ionization during operation, ensuring stable laser performance.
- Lighting: Noble gases are utilized in lighting applications, including neon signs and fluorescent lights, due to their unique spectral emission properties when excited. Their high ionization energies contribute to the stability and longevity of these light sources.
- Scientific Research: The inert nature of noble gases makes them valuable in various scientific experiments and processes. Their high ionization energies ensure minimal chemical reactivity, preventing unwanted side reactions.
Conclusion: High but Not Absent
Noble gases, despite their reputation for inertness, do indeed possess ionization energy. Their exceptionally high ionization energies reflect their exceptionally stable electronic configurations. While the general trend shows a decrease in ionization energy down the group due to increasing atomic radius and shielding, Helium stands as a notable exception. Understanding the factors influencing these ionization energies is crucial in various scientific and technological applications that leverage the unique properties of these elements. The high ionization energies are key to their inertness and their use in specialized applications requiring stability and resistance to chemical reactions. Further research continues to unveil the subtle nuances of electron behavior in these fascinating elements and their impact on their properties.
Latest Posts
Latest Posts
-
The Only Movable Joint In The Skull Is Between The
Mar 31, 2025
-
Which Inequality Is Shown In The Graph Below
Mar 31, 2025
-
Which Mrna Sequence Complements The Dna Sequence Below
Mar 31, 2025
-
Square Root Of 6 Is Irrational
Mar 31, 2025
-
A Record Is A Collection Of Related Tables
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Do Noble Gases Have Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
