How To Separate Salt And Sand
News Leon
Mar 30, 2025 · 5 min read
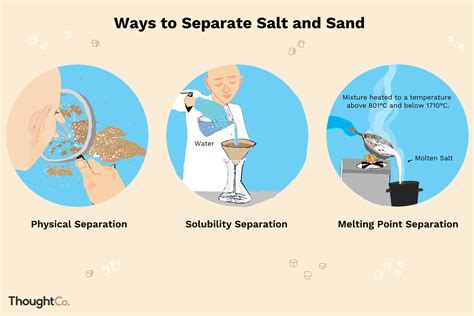
Table of Contents
How to Separate Salt and Sand: A Comprehensive Guide
Separating salt and sand might seem like a simple task, but it requires a deeper understanding of the physical and chemical properties of each substance to achieve effective separation. This comprehensive guide will explore various methods, from simple techniques suitable for home experiments to more advanced laboratory procedures, ensuring you gain a thorough understanding of the principles involved. We’ll delve into the science behind each method and highlight their advantages and disadvantages.
Understanding the Challenge: The Properties of Salt and Sand
Before diving into the separation techniques, it's crucial to understand the fundamental properties of salt (sodium chloride) and sand (primarily silicon dioxide). This understanding forms the basis for selecting the most appropriate separation method.
Salt's Properties:
- Solubility: Salt is highly soluble in water. This means it dissolves readily in water, forming a homogeneous solution. This property is the cornerstone of many separation techniques.
- Melting Point: Salt has a relatively high melting point (801°C), much higher than the boiling point of water.
- Density: Salt has a higher density than water (2.16 g/cm³ vs 1 g/cm³).
Sand's Properties:
- Insolubility: Sand is largely insoluble in water. This means it doesn't dissolve in water, remaining as a solid.
- Melting Point: Sand has an extremely high melting point (1710°C).
- Density: Sand has a higher density than water (approximately 2.65 g/cm³).
- Particle Size: Sand is composed of relatively large particles compared to salt crystals.
Methods for Separating Salt and Sand
Several methods can effectively separate salt and sand. The choice of method often depends on the scale of separation (e.g., a small amount for a science experiment versus a large industrial quantity) and the available resources.
1. Dissolution and Filtration: The Classic Method
This is perhaps the most common and straightforward method, leveraging the contrasting solubilities of salt and sand.
Procedure:
- Mix: Gently mix the salt and sand mixture.
- Dissolution: Add water to the mixture. The salt will dissolve, forming a saltwater solution, while the sand will remain undissolved. Stir thoroughly to ensure complete salt dissolution.
- Filtration: Use a filter paper (coffee filter, laboratory filter paper) and a funnel to separate the sand from the saltwater solution. The sand particles will be trapped by the filter paper, while the saltwater solution will pass through.
- Evaporation: Carefully pour the saltwater solution into a shallow dish or evaporating dish. Allow the water to evaporate slowly. As the water evaporates, the salt will be left behind as solid crystals.
Advantages: Simple, readily available materials, effective for small-scale separation.
Disadvantages: Time-consuming (especially the evaporation step), requires careful handling to avoid spills during filtration and evaporation. The purity of the recovered salt may be affected by any dissolved impurities in the water.
2. Density Separation: Leveraging Density Differences
This method utilizes the differences in the densities of salt, sand, and water. While less common for home experiments, it's valuable for understanding density-based separation techniques.
Procedure:
This method typically involves a series of steps using liquids of different densities. The exact procedure depends on the specific liquids used, but the general principle involves carefully layering liquids of increasing density, causing the sand and salt to separate based on their relative densities. This is often done using a separating funnel, allowing careful draining of the layers.
Advantages: Relatively quick, can achieve high purity if appropriate liquids are selected.
Disadvantages: Requires specialized equipment (separating funnel), needs careful selection of liquids with appropriate densities to ensure complete separation, potentially hazardous if using toxic liquids.
3. Decantation: A Simpler Approach
Decantation is a simpler technique that works well if you're comfortable with a slightly less pure salt recovery.
Procedure:
- Dissolution: Mix the salt and sand with water as described in the dissolution and filtration method.
- Sedimentation: Allow the mixture to settle for some time. The sand will settle to the bottom.
- Careful Pouring: Gently pour off the saltwater solution from the top, leaving most of the sand behind. Some sand might be carried over, resulting in less pure salt.
Advantages: Very simple, requires minimal equipment.
Disadvantages: Less efficient than filtration, resulting in less pure salt and potentially some loss of salt in the decantation process.
4. Advanced Techniques: Chromatography and Centrifugation
For highly precise separations or large-scale industrial applications, more advanced techniques are employed.
Chromatography: This technique exploits differences in the adsorption affinities of salt and sand to a stationary phase. While complex, it allows for extremely precise separation.
Centrifugation: This method uses centrifugal force to separate particles based on their size and density. While effective, it requires specialized equipment and isn't typically practical for home use.
These advanced methods are generally used in industrial settings or specialized laboratories where high purity and precision are crucial.
Troubleshooting Common Issues
Several challenges can arise during the separation process. Addressing these proactively can ensure successful separation.
- Incomplete Salt Dissolution: Ensure sufficient water is used and that the mixture is thoroughly stirred to dissolve the salt completely. Heating the water (carefully) can accelerate the dissolution process.
- Clogged Filter: Use a fine enough filter paper to prevent the passage of sand particles. If the filter clogs, consider using a new filter paper.
- Slow Evaporation: A shallow dish and a warm, dry environment will accelerate evaporation. Avoid direct sunlight to prevent the salt from being scattered.
- Impurities in the Water: Use distilled or purified water to minimize the contamination of the recovered salt.
Safety Precautions
When performing these experiments, always prioritize safety:
- Adult Supervision: Especially crucial for children.
- Heat Safety: Exercise caution when heating water. Avoid direct contact with hot liquids.
- Eye Protection: Consider wearing safety glasses, especially during the filtration and evaporation stages.
- Proper Disposal: Dispose of materials responsibly.
Conclusion: Mastering Salt and Sand Separation
Separating salt and sand effectively relies on a thorough understanding of the properties of both substances and the selection of appropriate separation techniques. From the simple dissolution and filtration method to more advanced techniques, the choice depends on the scale and desired purity. By carefully following the procedures and taking necessary safety precautions, you can successfully separate salt and sand, gaining a deeper appreciation for the principles of physical separation techniques. Remember to adapt the chosen method based on the quantity of the mixture and the available resources. This guide provides a foundational understanding, opening doors to more complex separation challenges in chemistry and other fields.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does Each Atom Of Arsenic Have
Apr 01, 2025
-
If I Drink I Die Riddle
Apr 01, 2025
-
Draw And Label A Ph Scale
Apr 01, 2025
-
Which Of The Following Are Characteristics Of A Competitive Market
Apr 01, 2025
-
An Indifference Curve Shows All Combinations Of Two Goods That
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How To Separate Salt And Sand . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
