How To Find The Molarity Of Naoh
News Leon
Mar 27, 2025 · 6 min read
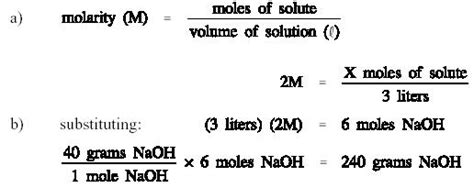
Table of Contents
How to Find the Molarity of NaOH: A Comprehensive Guide
Determining the molarity of a sodium hydroxide (NaOH) solution is a fundamental skill in chemistry, crucial for various applications, from titrations to preparing other solutions. Molarity, expressed as moles of solute per liter of solution (mol/L or M), represents the concentration of a solution. This guide provides a comprehensive explanation of how to find the molarity of NaOH, encompassing different methods and considerations.
Understanding Molarity and NaOH
Before diving into the methods, let's solidify our understanding of the core concepts:
What is Molarity?
Molarity (M) is a measure of the concentration of a solution. It's defined as the number of moles of solute (the substance being dissolved) present in one liter of the solution. The formula is:
Molarity (M) = Moles of solute (mol) / Volume of solution (L)
Sodium Hydroxide (NaOH): A Strong Base
Sodium hydroxide is a strong base, meaning it completely dissociates in water into sodium (Na⁺) and hydroxide (OH⁻) ions. This complete dissociation is crucial for accurate molarity calculations.
Methods for Determining NaOH Molarity
Several methods exist for determining the molarity of an NaOH solution. The most common approaches involve titration, using a known concentration of a standard solution to react with the NaOH. Let's explore these in detail:
Method 1: Titration with a Standard Acid Solution
This is the most accurate and widely used method. It involves reacting a known volume of the NaOH solution with a solution of a standard acid (an acid with a precisely known concentration), usually potassium hydrogen phthalate (KHP) or a standardized strong acid like HCl or H₂SO₄.
1. Preparing the Standard Acid Solution:
If you're starting with a solid acid like KHP, carefully weigh out a precise amount (e.g., 0.5 - 1 gram), dissolve it in distilled water, and dilute to a known volume in a volumetric flask. This gives you the precise molarity of the KHP solution using its molar mass. For liquid acids, using a standardized solution from a reliable source is critical for accuracy.
2. Performing the Titration:
- Set up: Fill a buret with the standard acid solution. Pipette a known volume (e.g., 25 mL) of the NaOH solution into an Erlenmeyer flask. Add a few drops of a suitable indicator, such as phenolphthalein (colorless in acidic solution, pink in basic solution).
- Titration process: Slowly add the standard acid solution from the buret to the NaOH solution, swirling constantly. The endpoint is reached when a single drop of acid causes a permanent color change (from pink to colorless with phenolphthalein).
- Record volumes: Carefully record the initial and final volumes of the standard acid solution from the buret. The difference represents the volume of acid used.
3. Calculating the Molarity of NaOH:
The stoichiometry of the reaction between the acid and base determines the molar ratio. For example, if using HCl (a monoprotic acid), the reaction is:
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
The mole ratio of NaOH to HCl is 1:1. We can use the following equation:
(Molarity of NaOH) x (Volume of NaOH) = (Molarity of acid) x (Volume of acid)
Solving for the molarity of NaOH:
Molarity of NaOH = [(Molarity of acid) x (Volume of acid)] / (Volume of NaOH)
Remember to convert all volumes to liters before calculating.
Example:
Let's say 25.00 mL of NaOH solution was titrated with 20.00 mL of 0.100 M HCl.
Molarity of NaOH = (0.100 M x 0.0200 L) / 0.0250 L = 0.0800 M
Therefore, the molarity of the NaOH solution is 0.0800 M.
Method 2: Titration with a Standard Base Solution (Indirect Method)
If you don't have a readily available standard acid, an indirect method using a standardized base is possible. This involves two titrations:
-
Titration of a known amount of a primary standard acid (like KHP) with the NaOH solution. This step determines the molarity of the NaOH relative to the KHP.
-
Titration of an unknown acid with the NaOH solution whose molarity has been determined in step 1. This step allows you to determine the concentration of the unknown acid.
This method involves more steps, but is useful when standard acid solutions aren't available.
Method 3: Using a Pre-Standardized NaOH Solution (Less Accurate)
Some suppliers offer pre-standardized NaOH solutions. While convenient, the accuracy depends heavily on the supplier's reliability and the storage conditions. Always check the certificate of analysis for the solution's exact concentration and expiry date. Note that NaOH solutions are hygroscopic (absorb moisture from the air), affecting their concentration over time.
Sources of Error and Precautions
Several factors can influence the accuracy of your molarity determination:
- Improperly standardized solutions: Using an inaccurately standardized acid or base will propagate errors into your calculation.
- Indicator error: Selecting an inappropriate indicator or failing to observe the endpoint accurately can lead to significant error.
- Parallax error: Incorrectly reading the buret meniscus can lead to volume measurement inaccuracies.
- Temperature fluctuations: Temperature changes can affect the volume of solutions, influencing molarity calculations.
- Impurities: Impurities in the NaOH or the standard acid can affect the results. Use high-purity reagents.
- Air exposure: NaOH solutions react with CO₂ in the air, forming sodium carbonate (Na₂CO₃), which alters the concentration of hydroxide ions. It is essential to minimize air exposure during the preparation and use of NaOH solutions. The use of a CO₂-free atmosphere might be necessary for ultra-precise measurements.
Minimizing Errors:
- Repeat the titration several times: This averages out random errors and improves the accuracy of your result.
- Use appropriate glassware: Employ calibrated volumetric flasks, pipettes, and burets for accurate volume measurements.
- Control temperature: Perform titrations at a consistent temperature to minimize volume errors.
- Careful technique: Follow proper laboratory techniques for handling chemicals and performing titrations.
- Use fresh solutions: Avoid using old or degraded solutions, as their concentrations may have changed.
Applications of Determining NaOH Molarity
Accurately determining the molarity of NaOH is vital in various chemical applications:
- Acid-base titrations: Determining the concentration of unknown acids or bases.
- Preparing standard solutions: Creating solutions with a precisely known concentration for various experiments.
- Synthesis reactions: In many chemical reactions, precise amounts of reactants are necessary, and NaOH molarity is a key factor in determining those amounts.
- pH control: NaOH is used to adjust the pH of solutions in many industrial and laboratory processes.
Conclusion
Determining the molarity of NaOH is a crucial skill in chemistry. While titration using a standardized acid remains the most accurate method, understanding the principles and potential sources of error is critical for obtaining reliable results. By carefully following the outlined steps and taking necessary precautions, you can accurately determine the molarity of your NaOH solution and confidently apply this knowledge to various chemical applications. Remember to always prioritize safety and proper laboratory techniques throughout the process. The accuracy of your results depends heavily on meticulous execution and attention to detail.
Latest Posts
Latest Posts
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 30, 2025
-
The Most Abundant Salt In Seawater Is
Mar 30, 2025
-
An Example Of An Externality Is The Impact Of
Mar 30, 2025
-
Which Of The Following Lines Is An Example Of Personification
Mar 30, 2025
-
17 Protons 18 Neutrons 17 Electrons
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Molarity Of Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
