Energy Required To Remove An Electron From A Gaseous Atom
News Leon
Mar 30, 2025 · 6 min read
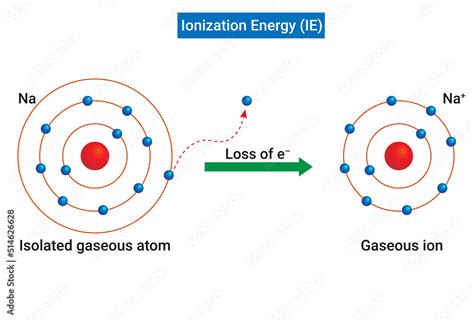
Table of Contents
The Energy Required to Remove an Electron from a Gaseous Atom: Ionization Energy
The energy required to remove an electron from a gaseous atom is a fundamental concept in chemistry and physics, known as ionization energy (IE), or ionization potential. Understanding ionization energy is crucial for comprehending atomic structure, chemical bonding, and the behavior of matter in various environments. This article delves deep into the intricacies of ionization energy, exploring its definition, trends across the periodic table, factors influencing its value, and its practical applications.
Defining Ionization Energy
Ionization energy is the minimum amount of energy needed to remove the most loosely bound electron from a neutral gaseous atom in its ground state. This process results in the formation of a positively charged ion, or cation. The equation representing the first ionization energy (IE<sub>1</sub>) is:
X(g) + energy → X<sup>+</sup>(g) + e<sup>-</sup>
Where:
- X(g) represents a neutral gaseous atom.
- X<sup>+</sup>(g) represents the resulting gaseous cation.
- e<sup>-</sup> represents the removed electron.
It's important to note that the atom must be in the gaseous state to avoid interatomic interactions that would affect the energy required for electron removal. Furthermore, the atom is considered to be in its ground state, meaning its electrons occupy the lowest possible energy levels.
Subsequent ionization energies (IE<sub>2</sub>, IE<sub>3</sub>, etc.) refer to the energy required to remove additional electrons from the already ionized atom. Each subsequent ionization energy is always greater than the preceding one because removing an electron from a positively charged ion requires overcoming the stronger electrostatic attraction between the remaining electrons and the increased positive nuclear charge.
Trends in Ionization Energy Across the Periodic Table
Ionization energy exhibits predictable trends across the periodic table, primarily influenced by two key factors: effective nuclear charge and atomic radius.
Effective Nuclear Charge
Effective nuclear charge (Z<sub>eff</sub>) refers to the net positive charge experienced by an electron in an atom. It's the difference between the actual nuclear charge (number of protons) and the shielding effect of inner electrons. As you move across a period (left to right), the number of protons increases, leading to a higher Z<sub>eff</sub>. This stronger attraction pulls the outermost electrons closer to the nucleus, requiring more energy to remove them. Therefore, ionization energy generally increases across a period.
Atomic Radius
Atomic radius refers to the size of an atom. As you move down a group (top to bottom), the number of electron shells increases, leading to a larger atomic radius. The increased distance between the outermost electrons and the nucleus reduces the electrostatic attraction, thus requiring less energy to remove them. Consequently, ionization energy generally decreases down a group.
Factors Influencing Ionization Energy
Besides effective nuclear charge and atomic radius, several other factors can influence ionization energy:
Electron Configuration
Electrons in filled subshells (s<sup>2</sup>, p<sup>6</sup>, d<sup>10</sup>, f<sup>14</sup>) are more stable and harder to remove than electrons in partially filled subshells. This leads to irregularities in the ionization energy trend across periods, particularly within the transition metals and post-transition metals. For example, the ionization energy of nitrogen is higher than oxygen because removing an electron from nitrogen would disrupt a stable half-filled p subshell.
Penetration Effect
Electrons in s orbitals penetrate closer to the nucleus than electrons in p, d, or f orbitals. This results in s electrons experiencing a higher effective nuclear charge and thus having higher ionization energies compared to electrons in other orbitals of the same shell.
Shielding Effect
Inner electrons shield the outer electrons from the full positive charge of the nucleus. The effectiveness of shielding depends on the type of orbital. s orbitals penetrate most effectively, while f orbitals shield least effectively. This difference in shielding contributes to variations in ionization energies.
Applications of Ionization Energy
Ionization energy is not just a theoretical concept; it has significant practical applications in various fields:
Spectroscopy
Ionization energy is directly related to the energy levels of electrons in atoms. Spectroscopic techniques, such as photoelectron spectroscopy (PES), measure the energy of electrons ejected from atoms upon absorption of photons. Analysis of these energy values allows determination of ionization energies and provides insights into atomic structure and electron configurations.
Chemistry
Ionization energy plays a crucial role in understanding chemical bonding. Atoms with low ionization energies readily lose electrons to form positive ions (cations), while atoms with high ionization energies tend to gain electrons to form negative ions (anions). This difference in electronegativity drives the formation of ionic bonds. The difference in ionization energies between two atoms also influences the nature of covalent bonds, determining the polarity and strength of the bond.
Materials Science
Ionization energy is essential in materials science for predicting and understanding the properties of materials. It influences the electrical conductivity, reactivity, and other physical and chemical characteristics of materials. For instance, materials with low ionization energies are often used as conductors, while those with high ionization energies are frequently used as insulators.
Astrophysics
Ionization energies are essential in astrophysics to analyze the composition of stars and other celestial objects. The analysis of light emitted or absorbed by celestial bodies provides information about the ionization states of atoms and molecules present, revealing information about the temperature, density, and chemical composition of these objects.
Medical Imaging
Ionization energy is crucial in techniques like X-ray imaging and computed tomography (CT scans). X-rays ionize atoms in the body, and the resulting signals are used to create images of internal structures. Understanding the ionization energies of different elements and their interactions with X-rays is essential for optimizing these imaging techniques.
Higher Ionization Energies and Electronic Structure
While the first ionization energy provides valuable information, subsequent ionization energies provide even more insight into electronic structure. The large jumps in ionization energies often indicate the removal of an electron from a different principal energy level or a filled subshell. Analyzing the pattern of ionization energies allows scientists to deduce the number of electrons in each shell and subshell, confirming the electronic configuration of an atom.
For example, consider the ionization energies of sodium (Na). The first ionization energy is relatively low, indicating the ease of removal of the single valence electron from the 3s orbital. However, the second ionization energy is significantly higher, demonstrating the increased difficulty of removing an electron from a stable, filled 2p subshell. This difference clearly illustrates the shell structure of the atom.
Conclusion
Ionization energy is a cornerstone concept in understanding atomic structure and behavior. Its predictable trends across the periodic table, along with its dependence on various factors like effective nuclear charge and electron configuration, provide powerful tools for predicting and explaining the properties of elements and their compounds. The wide-ranging applications of ionization energy in various scientific disciplines underscore its importance and its continued relevance in modern research. Further research into this fundamental property promises to yield deeper insights into the complexities of matter and its interactions. The relationship between ionization energy and other atomic properties, such as electron affinity and electronegativity, continues to be a vibrant area of study, enriching our understanding of the chemical world around us.
Latest Posts
Latest Posts
-
Weak Acid And Weak Base Ph
Apr 01, 2025
-
What Is The Measure Of Angle B In Degrees
Apr 01, 2025
-
What Is The Formula Of Iq
Apr 01, 2025
-
A Path That An Electric Current Follows Is A
Apr 01, 2025
-
Distance From Earth To Sun Scientific Notation
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Energy Required To Remove An Electron From A Gaseous Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
