The Most Abundant Salt In Seawater Is
News Leon
Mar 30, 2025 · 5 min read
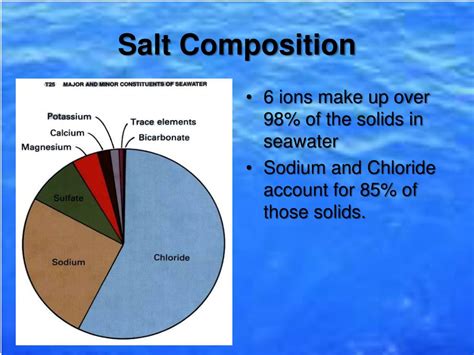
Table of Contents
The Most Abundant Salt in Seawater Is: Delving into Sodium Chloride and Ocean Salinity
The ocean's vastness holds a seemingly endless supply of dissolved substances, a complex cocktail of minerals and salts that shape marine life and influence global climate. While numerous salts contribute to the overall salinity of seawater, one reigns supreme: sodium chloride (NaCl), commonly known as table salt. Understanding the prevalence of sodium chloride, its origins, and its implications for marine ecosystems and the planet is crucial for appreciating the intricate workings of our oceans.
The Dominance of Sodium Chloride: A Quantitative Look
Seawater's salinity, typically expressed as parts per thousand (ppt), is a measure of the total dissolved salts in the water. This salinity varies geographically, influenced by factors like evaporation rates, freshwater inflow (from rivers and melting ice), and precipitation. However, despite these variations, sodium chloride consistently accounts for approximately 85% of the total dissolved salts in seawater. This makes it overwhelmingly the most abundant salt in the ocean. Other salts, such as magnesium chloride (MgCl₂), magnesium sulfate (MgSO₄), calcium sulfate (CaSO₄), and potassium chloride (KCl), contribute significantly to the remaining 15%, but their individual concentrations are considerably lower than that of sodium chloride.
The Chemical Composition of Seawater: A Detailed Breakdown
To understand the dominance of sodium chloride, let's consider a typical composition of seawater salinity:
- Sodium Chloride (NaCl): Approximately 85%
- Magnesium Chloride (MgCl₂): Approximately 10.8%
- Sodium Sulfate (Na₂SO₄): Approximately 4.7%
- Calcium Chloride (CaCl₂): Approximately 1.2%
- Potassium Chloride (KCl): Approximately 1.1%
- Other salts: Approximately 1.2% (This includes trace amounts of numerous other salts and minerals)
This composition demonstrates the clear supremacy of sodium chloride in contributing to the overall salinity of the ocean.
The Origins of Sodium Chloride in Seawater: A Geological Perspective
The accumulation of sodium chloride in the ocean is a result of a complex interplay of geological processes occurring over millions of years. These processes include:
-
Weathering and Erosion of Rocks: Rainfall and other forms of weathering break down rocks on land, releasing various ions, including sodium (Na⁺) and chloride (Cl⁻), into rivers and streams. These ions are then transported to the ocean via river runoff. This process is a continuous, ongoing source of dissolved salts in the ocean.
-
Hydrothermal Vents: Deep-sea hydrothermal vents release vast quantities of dissolved minerals, including sodium and chloride ions, into the ocean. These vents are located along mid-ocean ridges, where tectonic plates meet and magma rises to the seafloor.
-
Volcanic Activity: Volcanic eruptions release significant amounts of gases into the atmosphere, some of which contain chlorine compounds. These compounds can then dissolve in rainwater and eventually find their way into the ocean.
-
Sea Spray: Ocean waves generate sea spray, which carries fine droplets of seawater into the atmosphere. While some water evaporates, the salt remains in the air, eventually falling back to the ocean or land.
These processes continuously add sodium and chloride ions to the ocean, contributing to its high salinity.
The Significance of Sodium Chloride: Ecological and Climatic Implications
The abundance of sodium chloride in seawater profoundly impacts marine ecosystems and global climate patterns.
Marine Life and Salinity: A Delicate Balance
Seawater salinity is a crucial factor influencing the survival and distribution of marine organisms. Most marine organisms have adapted to thrive within a specific salinity range. Changes in salinity, even slight ones, can have significant consequences for their physiology, reproduction, and overall health. For example, changes in salinity can affect the osmotic balance of marine organisms, leading to dehydration or excessive water intake.
Salinity's Role in Ocean Currents and Climate
Ocean salinity plays a significant role in driving ocean currents. The differences in salinity (and temperature) between various parts of the ocean create density gradients, which drive thermohaline circulation—a global system of ocean currents that transports heat around the planet. This circulation is crucial for regulating global climate patterns, influencing weather systems, and distributing heat and nutrients throughout the ocean.
Sodium Chloride and Human Activities: A Growing Concern
Human activities significantly impact ocean salinity. For instance:
-
Coastal Development: Construction along coastlines can alter freshwater inflow into the ocean, potentially leading to localized salinity changes.
-
Desalination: The process of desalination, used to produce freshwater from seawater, generates concentrated brine, which, if not properly managed, can harm marine life.
-
Climate Change: Global climate change is altering precipitation patterns and sea ice melt, thereby affecting ocean salinity levels and potentially disrupting ocean currents.
These factors highlight the crucial importance of understanding and managing the impacts of human activities on the ocean's delicate salinity balance.
Beyond Sodium Chloride: The Importance of Other Salts in Seawater
While sodium chloride is the most abundant salt, other salts present in seawater play crucial roles in marine ecosystems and geological processes.
-
Magnesium Chloride (MgCl₂): Magnesium is an essential nutrient for many marine organisms, playing crucial roles in photosynthesis and skeletal formation.
-
Magnesium Sulfate (MgSO₄): This salt contributes to the bitterness of seawater and is essential for several physiological processes in marine organisms.
-
Calcium Sulfate (CaSO₄): This salt plays a vital role in the formation of calcium carbonate shells and skeletons in many marine invertebrates.
-
Potassium Chloride (KCl): Potassium is an essential nutrient for marine life, acting as a vital component of many cellular processes.
The interplay between these various salts determines the overall chemical characteristics of seawater and its ability to support life.
Conclusion: A Complex and Dynamic System
The most abundant salt in seawater is unequivocally sodium chloride, comprising approximately 85% of the total dissolved salts. However, the complete picture of ocean salinity involves a complex interplay of numerous salts and minerals, each contributing to the unique chemical composition of seawater. Understanding the sources, distribution, and significance of these salts is crucial for appreciating the intricate workings of ocean ecosystems and the global climate system. As human activities continue to impact our oceans, a deeper understanding of these dynamics becomes increasingly crucial for effective conservation and sustainable management of this vital resource. Further research focusing on the long-term impacts of climate change and human activities on ocean salinity is essential to ensuring the health and stability of our marine environment for future generations. The vast and mysterious ocean, with its complex chemistry, continues to reveal new insights, underscoring the importance of ongoing research and careful monitoring of this vital resource.
Latest Posts
Latest Posts
-
What Element Has 4 Protons And 5 Neutrons
Apr 01, 2025
-
Why Does Radius Decrease Across A Period
Apr 01, 2025
-
The Krebs Cycle Takes Place In
Apr 01, 2025
-
What Is The Following Sum In Simplest Form
Apr 01, 2025
-
Weak Acid And Weak Base Ph
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Most Abundant Salt In Seawater Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
