How Many Water Molecules In A Drop Of Water
News Leon
Mar 18, 2025 · 5 min read
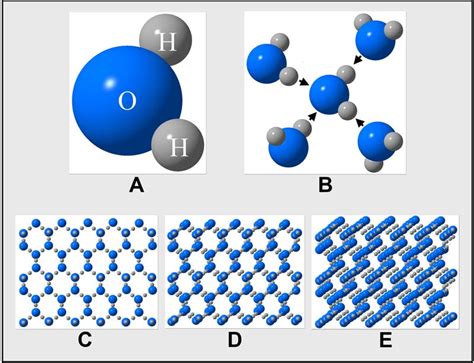
Table of Contents
How Many Water Molecules in a Drop of Water? A Deep Dive
Ever wondered about the sheer number of tiny particles making up something as seemingly simple as a single drop of water? It's a question that delves into the fascinating world of chemistry and the scale of the universe within the everyday. This article will explore exactly how many water molecules are in a drop of water, and the scientific principles that help us calculate this astonishing figure. We'll journey from basic concepts to complex calculations, demystifying the microscopic world and highlighting the significance of this seemingly simple question.
Understanding the Building Blocks: Water Molecules (H₂O)
Before we can estimate the number of water molecules, let's establish a foundational understanding of the water molecule itself. A water molecule (H₂O) is comprised of two hydrogen atoms covalently bonded to a single oxygen atom. This simple structure, however, exhibits remarkable properties that are crucial for life on Earth. The polarity of the water molecule, stemming from the difference in electronegativity between oxygen and hydrogen, leads to hydrogen bonding – a critical intermolecular force responsible for many of water's unique characteristics, including its high boiling point and surface tension.
Avogadro's Number: The Key to Counting Molecules
The key to unlocking the mystery of how many water molecules are in a drop lies in Avogadro's number. This fundamental constant in chemistry, approximately 6.022 x 10²³, represents the number of constituent particles (atoms, molecules, ions, etc.) in one mole of a substance. A mole is a unit of measurement in chemistry, much like a dozen (12) is a unit for counting eggs. The crucial point here is that one mole of any substance contains Avogadro's number of particles.
Defining a "Drop" of Water: The Challenge of Measurement
Before we can perform any calculations, we need to define what constitutes a "drop" of water. This seemingly straightforward task is surprisingly complex. The size of a drop depends significantly on several factors, including:
- Surface tension: The cohesive forces within water create surface tension, influencing the shape and size of a drop.
- The method of dispensing: A drop from an eyedropper will differ in size from a drop falling from a leaky faucet.
- Temperature and viscosity: Temperature affects the viscosity of water, indirectly affecting drop size.
- Environmental conditions: Humidity can impact the size and shape of a drop.
For our calculations, we'll need to make an assumption about the volume of a "drop." A commonly used approximation is 0.05 mL (milliliters). While this isn't universally precise, it serves as a reasonable starting point for our estimations. Keep in mind that the final number will vary depending on the assumed volume.
Calculating the Number of Water Molecules
Now we're ready to delve into the calculation. We will need to utilize several scientific concepts and units:
-
Density of Water: The density of water is approximately 1 g/mL (1 gram per milliliter). This means that 1 mL of water has a mass of 1 gram.
-
Molar Mass of Water: To determine the number of moles in our drop, we need the molar mass of water. The molar mass is the mass of one mole of a substance. For water (H₂O):
- Hydrogen (H) has an atomic mass of approximately 1 g/mol.
- Oxygen (O) has an atomic mass of approximately 16 g/mol.
Therefore, the molar mass of water is 1 g/mol (2H) + 16 g/mol (O) = 18 g/mol.
-
Converting Volume to Mass: Using the density of water, we can convert the volume of our drop (0.05 mL) to mass:
0.05 mL * 1 g/mL = 0.05 g
-
Converting Mass to Moles: We can now convert the mass of our drop to moles using the molar mass of water:
0.05 g / 18 g/mol = 0.00278 moles
-
Calculating the Number of Molecules: Finally, we can multiply the number of moles by Avogadro's number to determine the number of water molecules:
0.00278 moles * 6.022 x 10²³ molecules/mol ≈ 1.67 x 10²¹ molecules
Therefore, a 0.05 mL drop of water contains approximately 1.67 x 10²¹ water molecules. This is an incredibly large number – more than the number of grains of sand on all the beaches on Earth!
Variations and Considerations: The Importance of Precision
It's crucial to remember that this calculation is an approximation. The actual number of water molecules can vary depending on the precise volume of the drop and the assumptions made. Factors like temperature and pressure can also subtly influence the density of water and thus the final result. However, the order of magnitude (10²¹) remains consistent across reasonable variations in drop size.
The Impact of Impurities: Pure Water vs. Real-World Water
The calculation above assumes pure water. In reality, water typically contains dissolved ions, gases, and other impurities. These impurities will slightly affect the overall density and mass of the water, leading to a minor adjustment in the final count of water molecules. However, for most practical purposes, this effect remains negligible compared to the sheer number of water molecules present.
Conclusion: The Immensity of the Microscopic World
This exploration highlights the incredible scale of the microscopic world. A seemingly insignificant drop of water contains a truly astronomical number of molecules. Understanding this principle provides valuable insight into the fundamental nature of matter and the power of scientific tools and calculations in exploring the universe, even at its smallest scales. By utilizing Avogadro's number and basic principles of chemistry, we've unravelled the mystery of the countless water molecules within a single drop, demonstrating the beauty and complexity of the seemingly simple. This calculation is not just a mathematical exercise; it underscores the fundamental interconnectedness of seemingly disparate concepts, emphasizing the importance of precision in scientific measurement and analysis.
Latest Posts
Latest Posts
-
A Cell In A Hypertonic Solution Will
Mar 19, 2025
-
What Is 25 Percent Of 25
Mar 19, 2025
-
How Do You Make A Magnet At Home
Mar 19, 2025
-
A Decrease In Demand And An Increase In Supply Will
Mar 19, 2025
-
Prove The Square Root Of 5 Is Irrational
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Water Molecules In A Drop Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
