A Cell In A Hypertonic Solution Will
News Leon
Mar 19, 2025 · 6 min read
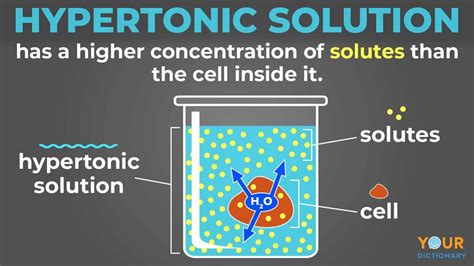
Table of Contents
A Cell in a Hypertonic Solution Will: Understanding Osmosis and its Effects
Understanding how cells behave in different environments is crucial to comprehending fundamental biological processes. One key concept is osmosis, the movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement aims to equalize the concentration of water on both sides of the membrane. When a cell is placed in a hypertonic solution, significant changes occur due to this osmotic pressure. Let's delve into the details of what happens to a cell in a hypertonic solution.
What is a Hypertonic Solution?
A hypertonic solution is one where the concentration of solutes (dissolved substances) is higher outside the cell than inside the cell. Water concentration, conversely, is higher inside the cell. Think of it like this: imagine a cell surrounded by a very salty solution. The salt is the solute, and the water is the solvent. Since the concentration of salt is higher outside, the concentration of water is lower outside. This difference in concentration drives the movement of water.
The Process of Osmosis in a Hypertonic Environment
When a cell is immersed in a hypertonic solution, water moves out of the cell across the cell membrane, attempting to balance the solute concentration. This outward movement of water causes the cell to shrink or crenate. This process is driven by the principle of osmosis: water moves down its concentration gradient, from an area of high concentration (inside the cell) to an area of low concentration (outside the cell).
The Role of the Cell Membrane
The cell membrane, also known as the plasma membrane, plays a vital role in this process. It is a selectively permeable membrane, meaning it allows some substances to pass through while restricting others. Water molecules can freely move across this membrane through aquaporins – specialized protein channels that facilitate water transport. However, larger solute molecules, like salts or sugars, typically cannot freely cross the membrane unless aided by specific transport proteins.
Factors Affecting Osmosis
Several factors can influence the rate of osmosis and the extent of cell shrinkage in a hypertonic solution:
-
The magnitude of the concentration gradient: The steeper the concentration gradient (the greater the difference in solute concentration between the inside and outside of the cell), the faster the rate of water movement.
-
The surface area of the cell membrane: A larger surface area allows for more water to move across the membrane simultaneously, accelerating the process.
-
The permeability of the cell membrane: A more permeable membrane allows for faster water movement.
-
Temperature: Higher temperatures generally increase the rate of osmosis as molecules have greater kinetic energy.
Effects on Different Cell Types
The effects of a hypertonic solution vary slightly depending on the type of cell:
Animal Cells
Animal cells lack a rigid cell wall. Consequently, when placed in a hypertonic solution, they undergo a significant change in shape and volume. The cell shrinks noticeably as water leaves, a process known as crenation. Severe crenation can damage the cell membrane and ultimately lead to cell death.
Examples of Crenation in Animal Cells
Think of a red blood cell placed in a highly concentrated salt solution. The water inside the red blood cell will move out into the solution, causing the cell to shrivel and become spiky in appearance. This is a classic example of crenation.
Plant Cells
Plant cells possess a rigid cell wall outside the cell membrane. This cell wall provides structural support and protection. When a plant cell is placed in a hypertonic solution, water moves out of the cell, causing the cell membrane to pull away from the cell wall. This process is called plasmolysis. While the cell loses turgor pressure (the pressure exerted by the cell contents against the cell wall), the cell wall prevents complete collapse of the cell.
Reversal of Plasmolysis
Interestingly, plasmolysis is reversible. If the plant cell is transferred back to a hypotonic or isotonic solution, water will re-enter the cell, causing the cell membrane to push back against the cell wall and restoring turgor pressure. This process is crucial for plant cell survival and function.
Bacterial Cells
Similar to plant cells, bacterial cells also possess a cell wall. When placed in a hypertonic solution, they also experience plasmolysis. Water leaves the cell, causing the cell membrane to detach from the cell wall. This can inhibit bacterial growth and even lead to bacterial cell death depending on the severity and duration of the hypertonic condition.
Significance of Hypertonic Solutions in Biology and Medicine
Understanding the effects of hypertonic solutions is vital across several biological and medical fields:
Food Preservation
Hypertonic solutions are used in food preservation techniques like salting or sugaring food items. The high solute concentration draws water out of microorganisms (like bacteria), inhibiting their growth and extending the shelf life of the food.
Medicine
Hypertonic solutions are used in some medical treatments. For example, intravenous administration of hypertonic saline solutions can help treat hyponatremia (low sodium levels in the blood) by drawing water from the cells into the bloodstream. Hypertonic solutions are also used in wound care to draw out excess fluid from wounds.
Plant Physiology
The understanding of plasmolysis and turgor pressure is crucial to studying plant physiology, especially in areas relating to water uptake, drought tolerance, and the overall health of plants.
Practical Applications and Examples
The effect of hypertonic solutions is demonstrable in numerous everyday scenarios:
-
Pickling: Pickles are preserved in a hypertonic brine solution (high salt concentration), which draws water out of the cucumber, preventing spoilage.
-
Fruit preservation: Fruits are preserved by making jams or jellies where a high sugar concentration acts as a hypertonic solution preventing microbial growth.
-
Meat curing: Salt and other ingredients create a hypertonic environment that dehydrates the meat, preventing microbial growth and enhancing flavor.
Conclusion: Understanding the Impact of Hypertonic Solutions
Understanding how cells respond to hypertonic solutions is fundamental to grasping many biological processes. The movement of water across the cell membrane, driven by osmosis, leads to either crenation in animal cells or plasmolysis in plant and bacterial cells. The consequences of these osmotic shifts range from reversible changes in cell shape and volume to irreversible damage and cell death. Knowledge of these principles is crucial in various fields, from food preservation to medical treatments, highlighting the pervasive influence of osmosis on biological systems. The significance extends beyond the cellular level, influencing plant health, microbial growth, and even human health applications. By understanding the intricate interplay between solute concentration, water movement, and cellular structure, we can appreciate the profound impact of hypertonic solutions on the living world.
Latest Posts
Latest Posts
-
Convert 59 Degrees Fahrenheit To Celsius
Mar 19, 2025
-
What Is 120 Hours In Days
Mar 19, 2025
-
How Many Cells Are In The Interphase
Mar 19, 2025
-
Packing Efficiency Of Body Centered Cubic
Mar 19, 2025
-
How Many Sig Figs Is 0 002
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about A Cell In A Hypertonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
