How Many Sig Figs Is 0.002
News Leon
Mar 19, 2025 · 5 min read
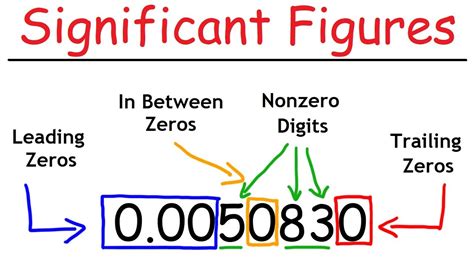
Table of Contents
How Many Significant Figures Are in 0.002? A Deep Dive into Significant Figures
The question, "How many significant figures are in 0.002?" seems deceptively simple. However, understanding significant figures (sig figs) requires a grasp of fundamental concepts in scientific notation and measurement accuracy. This article will not only answer this specific question but also provide a comprehensive guide to understanding significant figures, ensuring you can confidently tackle any similar problem.
What are Significant Figures?
Significant figures represent the number of digits in a value that contribute to its precision. They convey the reliability and uncertainty associated with a measurement. Simply put, they tell us how much we can trust the accuracy of a number. In scientific work, accurately reporting significant figures is crucial for maintaining the integrity and reproducibility of experimental results.
Why are Significant Figures Important?
The importance of significant figures stems from the inherent limitations of measurement. No measuring instrument is perfectly precise. Every measurement carries an inherent degree of uncertainty, and significant figures allow us to communicate this uncertainty clearly. Using too many significant figures implies a level of precision that doesn't exist, while using too few obscures the available information.
Rules for Determining Significant Figures
Several rules guide the determination of significant figures:
-
All non-zero digits are significant. For example, in the number 258, all three digits (2, 5, and 8) are significant.
-
Zeros between non-zero digits are significant. In the number 1005, all four digits are significant. The zeros are "sandwiched" between the 1 and the 5.
-
Leading zeros (zeros to the left of the first non-zero digit) are not significant. These zeros simply serve to place the decimal point. For example, in 0.002, the zeros before the 2 are not significant.
-
Trailing zeros (zeros to the right of the last non-zero digit) are significant only if the number contains a decimal point. In the number 100, only the 1 is significant. However, in 100., all three digits are significant. Similarly, 100.0 has four significant figures.
-
Trailing zeros in a number without a decimal point are ambiguous. The number 100 could have one, two, or three significant figures, depending on the precision of the measurement. Scientific notation avoids this ambiguity.
Applying the Rules to 0.002
Now, let's apply these rules to the number 0.002:
- Leading Zeros: The two zeros before the 2 are leading zeros. According to rule 3, they are not significant.
- Non-zero Digit: The digit 2 is a non-zero digit, and according to rule 1, it is significant.
Therefore, the number 0.002 has only one significant figure.
Understanding Uncertainty and Scientific Notation
The concept of significant figures is closely tied to the uncertainty inherent in measurements. Expressing numbers in scientific notation helps clarify the number of significant figures and the level of uncertainty. Scientific notation expresses a number as a value between 1 and 10 multiplied by a power of 10.
For instance, 0.002 in scientific notation is 2 x 10⁻³. This representation immediately highlights that only one digit (the 2) is significant. The exponent (-3) simply indicates the position of the decimal point.
Significant Figures in Calculations
The rules for significant figures extend to calculations involving multiplication, division, addition, and subtraction:
-
Multiplication and Division: The result should have the same number of significant figures as the measurement with the fewest significant figures.
-
Addition and Subtraction: The result should have the same number of decimal places as the measurement with the fewest decimal places.
Examples of Significant Figures in Calculations
Let's illustrate these rules with a few examples:
Multiplication:
12.34 (4 sig figs) x 2.1 (2 sig figs) = 25.914
The correct answer, considering significant figures, is 26 (2 sig figs).
Division:
25.914 / 12.34 = 2.0954...
The correct answer is 2.1 (2 sig figs).
Addition:
12.345 (5 sig figs) + 1.2 (1 decimal place) = 13.545
The correct answer is 13.5 (1 decimal place).
Subtraction:
15.78 (2 decimal places) – 3.2 (1 decimal place) = 12.58
The correct answer is 12.6 (1 decimal place).
Rounding and Significant Figures
When rounding numbers to the correct number of significant figures, follow these rules:
- If the digit to be dropped is less than 5, round down.
- If the digit to be dropped is greater than or equal to 5, round up.
For example, rounding 12.345 to three significant figures results in 12.3. Rounding 12.355 to three significant figures results in 12.4.
Ambiguity and Best Practices
While the rules for determining significant figures are relatively straightforward, ambiguity can still arise. For instance, the number 100 might represent a single significant figure if it's a rounded-off measurement of roughly 100, or it could represent three significant figures if the measurement was precisely 100. To avoid this ambiguity, always use scientific notation, expressing the number with the correct number of significant figures and clearly indicating the uncertainty.
Conclusion: Mastering Significant Figures for Accurate Scientific Communication
Significant figures are an essential aspect of scientific reporting and calculations. They serve as a vital tool for clearly expressing the level of precision and uncertainty associated with measurements and calculations. By consistently applying the rules outlined in this guide and employing scientific notation where appropriate, you can ensure the accuracy and integrity of your scientific work. Remembering that 0.002 has only one significant figure is not just about following a rule; it's about communicating the precision of your data responsibly and accurately within the scientific community. A firm grasp of significant figures is a fundamental skill for any student or professional working in science, engineering, or any field that relies on precise measurements and calculations.
Latest Posts
Latest Posts
-
How Many Seconds Is One Hour
Mar 19, 2025
-
The Solubility Of A Solute Depends On
Mar 19, 2025
-
The Complete Set Of Genes In An Organism
Mar 19, 2025
-
Formula Of Coefficient Of Kinetic Friction
Mar 19, 2025
-
What Are The Three Parts Of The Atp Molecule
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Sig Figs Is 0.002 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
