How Many Valence Electrons In Br
News Leon
Mar 14, 2025 · 5 min read
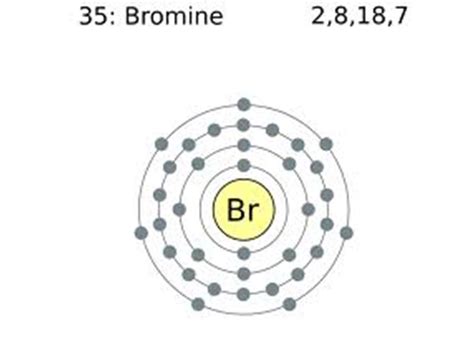
Table of Contents
How Many Valence Electrons Does Bromine (Br) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Bromine (Br), a fascinating reddish-brown liquid element, plays a crucial role in various chemical reactions and industrial processes. Understanding its properties, particularly its valence electrons, is key to comprehending its behavior and reactivity. This comprehensive article delves into the intricacies of bromine's atomic structure, explains the concept of valence electrons, and explores their significance in determining bromine's chemical behavior. We'll also touch upon related concepts like electron configuration, Lewis dot structures, and the implications for chemical bonding.
Understanding Atomic Structure: The Foundation of Valence Electrons
Before we determine the number of valence electrons in bromine, let's revisit the fundamental principles of atomic structure. Atoms are composed of a central nucleus containing positively charged protons and neutral neutrons. Surrounding the nucleus are negatively charged electrons, arranged in specific energy levels or shells. These shells are further subdivided into subshells (s, p, d, f), each capable of holding a specific number of electrons.
The arrangement of electrons in these shells and subshells is described by the electron configuration. This configuration dictates an atom's chemical properties and its ability to form bonds with other atoms. The outermost shell of an atom is particularly important because it houses the valence electrons.
Electron Configuration of Bromine (Br)
Bromine's atomic number is 35, meaning it has 35 protons and 35 electrons in a neutral atom. The electron configuration of bromine is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵.
This configuration reveals the distribution of electrons across different energy levels and subshells. Let's break it down:
- 1s²: Two electrons in the first shell (s subshell).
- 2s²2p⁶: Eight electrons in the second shell (two in the s subshell, six in the p subshell).
- 3s²3p⁶: Eight electrons in the third shell (two in the s subshell, six in the p subshell).
- 4s²3d¹⁰4p⁵: Seventeen electrons in the fourth shell (two in the 4s subshell, ten in the 3d subshell, and five in the 4p subshell).
Identifying Valence Electrons in Bromine
The valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound and are directly involved in chemical bonding. They determine an atom's reactivity and the types of bonds it can form (ionic, covalent, metallic).
In bromine's electron configuration (1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵), the outermost shell is the fourth shell (n=4). This shell contains seven electrons (two in the 4s subshell and five in the 4p subshell). Therefore, bromine has seven valence electrons.
Significance of Valence Electrons: Chemical Bonding and Reactivity
The seven valence electrons in bromine dictate its chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gas configuration (eight valence electrons, the octet rule). Bromine, being one electron short of a full octet, readily accepts an electron to achieve stability. This explains its high reactivity and tendency to form stable compounds.
Bromine's Chemical Bonding
Bromine's chemical bonding behavior is strongly influenced by its seven valence electrons. Bromine commonly forms:
-
Covalent bonds: Bromine shares its valence electrons with other atoms to achieve a stable octet. Examples include hydrogen bromide (HBr), bromine monochloride (BrCl), and numerous organic bromine compounds.
-
Ionic bonds: Although less common, bromine can accept an electron from a highly electropositive metal, forming a bromide ion (Br⁻) with a stable octet. This leads to the formation of ionic compounds like sodium bromide (NaBr) and potassium bromide (KBr).
Lewis Dot Structures: Visualizing Valence Electrons
Lewis dot structures are a simple yet powerful tool for visualizing valence electrons and predicting bonding patterns. In a Lewis structure, the element's symbol represents the nucleus and inner electrons, while dots surrounding the symbol represent the valence electrons.
The Lewis dot structure for bromine is:
. . . . .
:Br.
. .
This structure clearly shows bromine's seven valence electrons. When forming bonds, these dots are paired up to represent shared electron pairs in covalent bonds or transferred to another atom in ionic bonds.
Bromine's Role in Chemistry and Industry
Bromine's unique properties, primarily stemming from its seven valence electrons and high reactivity, lead to its diverse applications in various fields:
-
Flame retardants: Organobromine compounds are used as flame retardants in textiles, plastics, and electronics. This property is due to bromine's ability to disrupt combustion processes.
-
Water purification: Bromine compounds are used as disinfectants and sanitizers in water treatment. They effectively kill bacteria and other harmful microorganisms.
-
Agricultural chemicals: Bromine-containing compounds find applications in pesticides and fungicides. Their effectiveness stems from their ability to disrupt the metabolic processes of pests and fungi.
-
Pharmaceuticals: Bromine compounds are present in some pharmaceuticals, serving various roles, such as sedatives and anticonvulsants.
-
Photography: Silver bromide (AgBr) is a crucial component in photographic film and paper, acting as a light-sensitive material.
Conclusion: Understanding Bromine's Reactivity through its Valence Electrons
The number of valence electrons is a fundamental property that dictates an element's chemical behavior. Bromine, with its seven valence electrons, exhibits a high degree of reactivity, readily forming covalent and ionic bonds to achieve a stable octet. This reactivity is the foundation of bromine's vast applications across diverse industries. Understanding the link between its atomic structure, valence electron count, and chemical bonding helps in appreciating the crucial role bromine plays in our daily lives, from flame retardants to medical applications. By understanding bromine's seven valence electrons, we unlock a deeper understanding of its chemical properties and its significance in the world around us. Further investigation into bromine's reactivity and its interaction with other elements unveils more intricate details about its complex and fascinating chemistry.
Latest Posts
Latest Posts
-
Cells Placed In A Hypertonic Solution Will
Mar 14, 2025
-
Which Of The Following Is A Site For Lipid Synthesis
Mar 14, 2025
-
What Are Monomers Of Nucleic Acids
Mar 14, 2025
-
What Is The Electron Configuration Of Vanadium
Mar 14, 2025
-
Water Is Called Universal Solvent Why
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Br . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
