What Is The Electron Configuration Of Vanadium
News Leon
Mar 14, 2025 · 6 min read
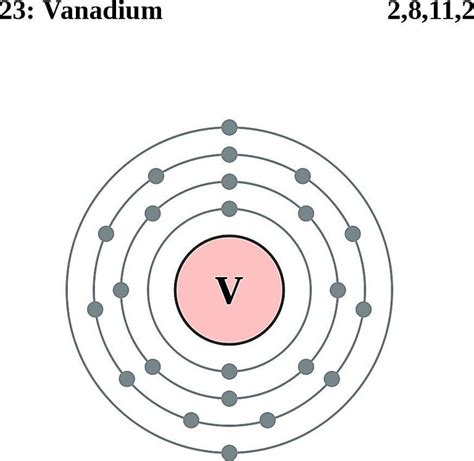
Table of Contents
What is the Electron Configuration of Vanadium? A Deep Dive into the Element's Atomic Structure
Vanadium, a transition metal with the symbol V and atomic number 23, holds a fascinating position in the periodic table. Its unique electron configuration is key to understanding its diverse chemical properties and applications. This article will provide a comprehensive exploration of vanadium's electron configuration, explaining the principles behind it, its implications for vanadium's reactivity, and its role in various fields.
Understanding Electron Configuration
Before diving into vanadium's specific configuration, let's establish a foundational understanding of what electron configuration represents. An electron configuration describes the arrangement of electrons in the different energy levels (shells) and sublevels (subshells) within an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and influencing its overall properties. The electron configuration follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This is further refined by Hund's rule, which dictates that electrons will individually occupy orbitals within a subshell before pairing up, and the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same four quantum numbers.
Shells, Subshells, and Orbitals
To understand electron configuration properly, we need to understand the terms:
-
Shells: These represent the principal energy levels, designated by the principal quantum number (n), where n = 1, 2, 3, and so on. Higher n values represent shells further from the nucleus and higher energy levels.
-
Subshells: Within each shell are subshells, designated by the azimuthal quantum number (l). For a given n, l can have values from 0 to n-1. These subshells are labeled s (l=0), p (l=1), d (l=2), and f (l=3). Each subshell has a specific number of orbitals.
-
Orbitals: Orbitals are regions within a subshell where there is a high probability of finding an electron. Each orbital can hold a maximum of two electrons with opposite spins. The s subshell has one orbital, the p subshell has three orbitals, the d subshell has five orbitals, and the f subshell has seven orbitals.
Determining Vanadium's Electron Configuration
Vanadium (V) has an atomic number of 23, meaning it has 23 protons and, in a neutral atom, 23 electrons. To determine its electron configuration, we follow the Aufbau principle, filling the lowest energy levels first:
1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>3</sup>
Let's break this down:
-
1s<sup>2</sup>: The first shell (n=1) contains the s subshell, which can hold up to two electrons. Both are filled.
-
2s<sup>2</sup>: The second shell (n=2) starts with the s subshell, again holding two electrons.
-
2p<sup>6</sup>: The second shell also contains the p subshell, which has three orbitals and can hold up to six electrons. All three orbitals are filled.
-
3s<sup>2</sup>: The third shell (n=3) begins with the s subshell, holding two electrons.
-
3p<sup>6</sup>: The third shell's p subshell, also filled to its capacity of six electrons.
-
4s<sup>2</sup>: The fourth shell (n=4) starts with the s subshell, filled with two electrons. Notice that the 4s subshell fills before the 3d subshell. This is because the 4s subshell has a slightly lower energy level than the 3d subshell.
-
3d<sup>3</sup>: Finally, we reach the 3d subshell in the third shell. Vanadium has three electrons remaining, which fill three of the five orbitals in the 3d subshell. According to Hund's rule, these electrons will occupy separate orbitals with parallel spins before pairing up.
Therefore, the complete electron configuration of vanadium is 1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>3</sup>. This configuration is crucial in determining vanadium's chemical behavior.
Implications of Vanadium's Electron Configuration
Vanadium's electron configuration, specifically the presence of three electrons in the 3d subshell, explains several of its key properties:
-
Variable Oxidation States: The 3d electrons are relatively loosely held and can participate in chemical bonding. This allows vanadium to exhibit multiple oxidation states, ranging from +2 to +5. This versatility is responsible for the wide range of vanadium compounds and their diverse applications.
-
Transition Metal Properties: The presence of incompletely filled d orbitals is characteristic of transition metals. Vanadium showcases many typical transition metal properties, including variable oxidation states, colored compounds, and catalytic activity. The d electrons are responsible for the formation of coloured complexes due to d-d electronic transitions.
-
Paramagnetism: The unpaired electrons in the 3d subshell make vanadium paramagnetic, meaning it is attracted to magnetic fields. This is a consequence of the electron spins.
-
Alloy Formation: Vanadium readily forms alloys with other metals, contributing to enhanced strength, toughness, and other desirable mechanical properties. Its electron configuration contributes to its ability to form strong metallic bonds with other transition metals.
Applications of Vanadium and its Compounds
Vanadium's unique properties, stemming directly from its electron configuration, lead to its use in various applications:
-
Steel Alloys: Vanadium is a crucial alloying element in high-strength steels, enhancing their toughness and hardenability. The addition of vanadium significantly improves the mechanical properties of steel, making it suitable for demanding applications like tools and structural components.
-
Titanium Alloys: In the aerospace industry, vanadium is added to titanium alloys to enhance their strength and corrosion resistance. These alloys are prized for their high strength-to-weight ratio.
-
Vanadium Oxide Catalysts: Vanadium oxides are used as catalysts in various chemical processes, including the production of sulfuric acid and the oxidation of organic compounds. The variable oxidation states of vanadium allow it to participate in redox reactions effectively.
-
Vanadium Redox Flow Batteries: Vanadium redox flow batteries are gaining traction as a promising energy storage solution. These batteries utilize vanadium compounds in different oxidation states to store and release energy.
-
Biological Roles: Although less common, vanadium also plays a minor role in some biological systems, exhibiting enzymatic functions in certain organisms. This highlights the versatility of its chemical behaviour arising from its electronic configuration.
Conclusion: A Deeper Understanding of Vanadium
The electron configuration of vanadium, 1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>3</sup>, is fundamental to understanding its properties and its wide range of applications. The presence of three unpaired d electrons directly influences its variable oxidation states, paramagnetism, and its ability to form strong alloys and act as a catalyst. This seemingly simple arrangement of electrons underpins the important roles vanadium plays in materials science, energy storage, and even certain biological processes. Further research into vanadium's chemistry continues to uncover its potential in diverse fields, reaffirming the importance of understanding its atomic structure. The interplay between its electron configuration and its macroscopic properties serves as a perfect illustration of the power of quantum mechanics in explaining the world around us.
Latest Posts
Latest Posts
-
What Is Needed For Photosynthesis To Occur
Mar 14, 2025
-
A Rock Is Thrown Vertically Upward
Mar 14, 2025
-
The Production Possibilities Frontier Will Shift Outward
Mar 14, 2025
-
The Heart Chamber With The Thickest Wall Is The
Mar 14, 2025
-
What Is The Ultimate Goal Of A Political Party
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Vanadium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
