Water Is Called Universal Solvent Why
News Leon
Mar 14, 2025 · 6 min read
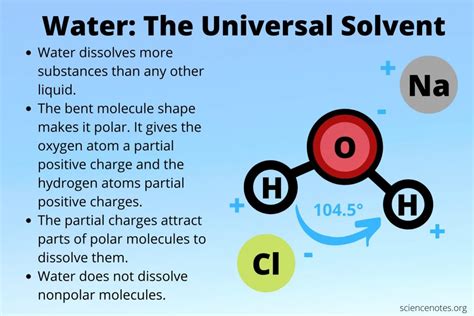
Table of Contents
Water: The Universal Solvent – Why It Dissolves So Much
Water, the elixir of life, is more than just a simple molecule; it's a remarkable substance with unique properties that underpin its crucial role in all known life forms. One of its most significant characteristics is its ability to act as a universal solvent, dissolving a wider range of substances than any other liquid. But why? What inherent properties of water contribute to this exceptional dissolving power? This article delves deep into the science behind water's solvency, exploring the molecular structure, polarity, and hydrogen bonding that make it the unparalleled solvent it is.
The Polarity of Water: A Key to Its Solvency
At the heart of water's solvency lies its polarity. A water molecule (H₂O) consists of two hydrogen atoms covalently bonded to a single oxygen atom. Oxygen is significantly more electronegative than hydrogen, meaning it attracts the shared electrons in the covalent bonds more strongly. This unequal sharing of electrons creates a polar molecule, with a slightly negative charge (δ-) near the oxygen atom and slightly positive charges (δ+) near the hydrogen atoms. This charge separation is represented as a dipole moment.
This inherent polarity is crucial because it allows water molecules to interact effectively with other polar molecules and ions. The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atoms of neighboring molecules, leading to the formation of hydrogen bonds. These are relatively weak bonds compared to covalent bonds, but their collective effect is powerful.
How Polarity Facilitates Dissolution
Think of dissolving table salt (NaCl) in water. NaCl is an ionic compound, consisting of positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). When salt is added to water, the polar water molecules surround the ions. The slightly negative oxygen atoms are attracted to the positively charged sodium ions, while the slightly positive hydrogen atoms are attracted to the negatively charged chloride ions. This process, known as hydration, effectively shields the ions from each other, preventing them from re-forming the crystal lattice and keeping them dissolved in the solution.
This same principle applies to many other polar substances. Sugars, for example, contain numerous hydroxyl (-OH) groups, which are polar and can form hydrogen bonds with water molecules, leading to their solubility. Similarly, many other polar molecules, such as alcohols and acids, readily dissolve in water due to the formation of hydrogen bonds.
Hydrogen Bonding: The Force Behind Water's Power
Hydrogen bonds are not unique to water; they occur in other molecules containing hydrogen atoms bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine. However, the abundance and strength of hydrogen bonds in water are exceptional. Each water molecule can form up to four hydrogen bonds with neighboring molecules, creating a complex, three-dimensional network. This network is highly dynamic, with bonds constantly breaking and reforming, contributing to the fluidity of water.
The Impact of Hydrogen Bonds on Solvency
The extensive hydrogen bonding network in water has profound implications for its solvency. It provides the energy needed to overcome the attractive forces between solute molecules, allowing them to become dispersed in the solvent. Moreover, the hydrogen bonds help to stabilize the dissolved ions or molecules, preventing them from re-aggregating. The strength and number of hydrogen bonds formed play a significant role in determining the solubility of a substance in water.
The Role of Water's High Dielectric Constant
Water's high dielectric constant also contributes significantly to its solvency. The dielectric constant is a measure of a substance's ability to reduce the electrostatic forces between charged particles. Water has an exceptionally high dielectric constant, meaning it effectively weakens the attractive forces between ions in an ionic compound, making it easier for the ions to become separated and dissolved.
This high dielectric constant helps to overcome the strong electrostatic attraction between the oppositely charged ions in ionic compounds like salt, facilitating the dissolution process. Without this high dielectric constant, the attractive forces between ions would be too strong, preventing them from being effectively separated by water molecules.
Limitations of Water's Universal Solvency
While water is a remarkable solvent, it's not truly a "universal" solvent in the strictest sense. There are many substances that are insoluble or only slightly soluble in water. These typically include:
-
Nonpolar substances: Substances like oils and fats are composed of nonpolar molecules, meaning they lack significant charge separation. Water molecules cannot effectively interact with nonpolar molecules, resulting in low solubility. The strong hydrogen bonding within water itself favors interactions between water molecules over interactions with nonpolar molecules. This phenomenon is often described as "like dissolves like."
-
Large, complex molecules: Even some polar molecules with high molecular weight may exhibit low solubility in water due to their size and complexity. The interaction between water molecules and the extensive surface area of these large molecules may not be sufficient to overcome the intermolecular forces within the solute.
-
Substances with strong intermolecular forces: Some substances have strong intermolecular forces (e.g., covalent bonds, strong dipole-dipole interactions) that are stronger than the attractive forces between water molecules and the solute. In such cases, water cannot effectively overcome these strong intermolecular forces, leading to low solubility.
The Importance of Water's Solvency to Life
The exceptional solvency of water is absolutely fundamental to life as we know it. Water's ability to dissolve a wide range of substances allows it to act as a:
-
Medium for biochemical reactions: Many biochemical reactions occur in aqueous solutions, where water acts as a solvent for reactants, products, and enzymes.
-
Transport medium: Water transports nutrients, hormones, and waste products throughout the body in blood and other bodily fluids.
-
Temperature regulator: Water's high heat capacity helps to regulate body temperature, preventing drastic fluctuations.
-
Lubricant and cushion: Water acts as a lubricant in joints and cushions organs, protecting them from damage.
Conclusion: Water's Unique Solvency - A Result of its Properties
Water's ability to act as a universal solvent is a direct consequence of its unique molecular structure, polarity, hydrogen bonding, and high dielectric constant. These properties combine to create a solvent capable of dissolving a vast array of substances, enabling the countless chemical reactions that sustain life. While not truly universal in dissolving every substance, its exceptional solvency makes it the cornerstone of life's processes on Earth. Understanding this remarkable property is critical to appreciating the fundamental role of water in biology, chemistry, and countless other scientific fields. Further research continues to explore the nuances of water's solvency, unveiling ever more intricate details of this essential molecule's behavior and influence on the world around us.
Latest Posts
Latest Posts
-
Which Element Is Found In All Organic Compounds
Mar 14, 2025
-
What Is The Largest Single Mass Of Lymphatic Tissue
Mar 14, 2025
-
How Many Radians Is One Revolution
Mar 14, 2025
-
This Reflex Arc Shows A
Mar 14, 2025
-
In Bacteria Photosynthetic Pigments Are Found In
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Water Is Called Universal Solvent Why . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
