Which Element Is Found In All Organic Compounds
News Leon
Mar 14, 2025 · 6 min read
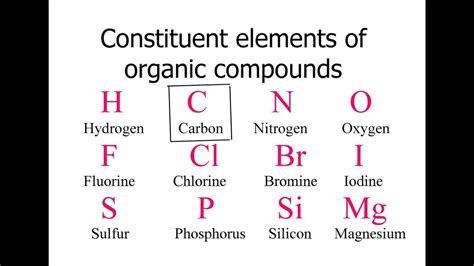
Table of Contents
Which Element is Found in All Organic Compounds?
The answer is simple, yet profound: carbon. Every organic compound contains carbon. This seemingly straightforward statement underpins an entire branch of chemistry, encompassing the study of millions of molecules fundamental to life itself and countless synthetic materials shaping our modern world. This article will delve deep into the unique properties of carbon that make it the cornerstone of organic chemistry, exploring its bonding capabilities, the diversity of organic compounds it forms, and its crucial role in biological systems and synthetic materials.
The Uniqueness of Carbon
Carbon's central role in organic chemistry stems from its exceptional ability to form stable covalent bonds. Unlike many other elements, carbon readily bonds with itself and a wide range of other atoms, including hydrogen, oxygen, nitrogen, sulfur, phosphorus, and halogens. This versatility allows carbon to construct an astonishing variety of molecular structures, from simple linear chains to complex branched structures, rings, and three-dimensional networks.
Carbon's Tetravalency
The heart of carbon's versatility lies in its tetravalency. A carbon atom has four valence electrons, meaning it can form four covalent bonds with other atoms. These bonds can be single, double, or triple bonds, leading to diverse molecular geometries and functionalities. The combination of single, double, and triple bonds enables the creation of a vast array of structural isomers – molecules with the same chemical formula but different arrangements of atoms.
Carbon's Ability to Catinate
Another critical property is carbon's remarkable capacity for catenation, the ability to form long chains and rings by bonding with other carbon atoms. This self-linking ability is unparalleled among the elements, allowing for the construction of macromolecules, including polymers, proteins, and nucleic acids. The chains can be linear, branched, or cyclic, leading to further complexity and diversity.
Hybridization and Molecular Geometry
The way carbon bonds also affects the shape of molecules. Carbon atoms can undergo hybridization, a process where atomic orbitals mix to form hybrid orbitals. The most common types are sp³, sp², and sp hybridization, resulting in tetrahedral, trigonal planar, and linear geometries, respectively. These variations in geometry further contribute to the immense diversity of organic compounds.
The Diversity of Organic Compounds
The consequence of carbon's unique properties is the staggering diversity of organic compounds. Millions of organic compounds are known, and new ones are being discovered and synthesized constantly. This vastness can be categorized into several major classes, each with its own characteristic properties and functions:
Hydrocarbons
Hydrocarbons are the simplest organic compounds, consisting solely of carbon and hydrogen atoms. They are the foundation upon which more complex organic molecules are built. Hydrocarbons can be classified as:
-
Alkanes: These are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. They are relatively unreactive but form the basis for many other organic compounds. Examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈).
-
Alkenes: These are unsaturated hydrocarbons containing at least one carbon-carbon double bond. The double bond introduces reactivity and the potential for geometric isomerism (cis-trans isomerism). Ethylene (C₂H₄) is a common example.
-
Alkynes: These are unsaturated hydrocarbons containing at least one carbon-carbon triple bond. The triple bond confers even greater reactivity than a double bond. Acetylene (C₂H₂) is a well-known example.
-
Aromatic Hydrocarbons: These hydrocarbons contain a benzene ring, a six-carbon ring with alternating single and double bonds. The delocalized electrons in the benzene ring give these compounds unique properties and stability. Benzene (C₆H₆) is the simplest aromatic hydrocarbon.
Functional Groups
Beyond hydrocarbons, the vast majority of organic compounds contain functional groups. These are specific groups of atoms within a molecule that confer characteristic chemical properties. Common functional groups include:
-
Alcohols (-OH): Containing a hydroxyl group, alcohols are polar and can participate in hydrogen bonding.
-
Aldehydes (-CHO): Containing a carbonyl group at the end of a carbon chain, aldehydes are often found in fragrances and flavors.
-
Ketones (-C=O): Containing a carbonyl group within a carbon chain, ketones are often used as solvents.
-
Carboxylic Acids (-COOH): Containing a carboxyl group, carboxylic acids are acidic and participate in many biological processes.
-
Esters (-COO-): Often formed from carboxylic acids and alcohols, esters frequently have pleasant aromas and are used in perfumes and flavorings.
-
Amines (-NH₂): Containing an amino group, amines are basic and important components of proteins.
-
Amides (-CONH₂): Containing an amide group, amides are crucial components of proteins and peptides.
The Role of Carbon in Biological Systems
Carbon's ability to form diverse and complex molecules is fundamental to life. All major classes of biomolecules – carbohydrates, lipids, proteins, and nucleic acids – are based on carbon backbones.
Carbohydrates
Carbohydrates, like glucose and starch, are crucial energy sources. Their structures are built around chains of carbon atoms linked to oxygen and hydrogen.
Lipids
Lipids, including fats and oils, are vital for energy storage, cell membranes, and hormone production. Their structures often involve long hydrocarbon chains.
Proteins
Proteins are the workhorses of cells, catalyzing reactions, transporting molecules, and providing structural support. Their structures are incredibly complex, built from chains of amino acids linked by peptide bonds, where carbon plays a vital role in the peptide backbone and side chains.
Nucleic Acids
Nucleic acids, like DNA and RNA, store and transmit genetic information. Their structures are built around a sugar-phosphate backbone and nitrogenous bases, all containing carbon atoms.
Carbon in Synthetic Materials
The versatility of carbon also extends to the realm of synthetic materials. Many materials crucial to modern society are based on carbon-containing compounds:
-
Polymers: Plastics, rubbers, and fibers are all based on carbon-containing polymers, long chains of repeating monomer units. These materials exhibit diverse properties depending on the monomers and their arrangement.
-
Pharmaceuticals: Many medicines are organic compounds designed to interact with specific biological targets. The carbon framework is essential for shaping these molecules' three-dimensional structure, enabling interaction with biological receptors.
-
Fuels: Fossil fuels, like coal, oil, and natural gas, are primarily composed of hydrocarbons. These fuels provide a significant portion of the world's energy.
Conclusion
In summary, carbon is the cornerstone element of organic chemistry. Its unique ability to form stable covalent bonds, its tetravalency, and its capacity for catenation give rise to an immense diversity of molecules with diverse properties and functions. The importance of carbon stretches across all aspects of life, from the basic building blocks of biological systems to the synthetic materials that shape our modern world. Understanding the properties of carbon and the molecules it forms is essential to comprehending the complexities of both the natural and synthetic worlds. The ongoing exploration and innovation in organic chemistry continue to unveil new possibilities, further highlighting the unparalleled importance of carbon in the fabric of our universe.
Latest Posts
Latest Posts
-
Convert Timestamp To Date Time Python
Mar 14, 2025
-
Difference Between A Monologue And A Soliloquy
Mar 14, 2025
-
Coordination Number Of Body Centered Cubic
Mar 14, 2025
-
Integral Of 1 X 2 3
Mar 14, 2025
-
How Many Cubic Centimeters In 1 Cubic Meter
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Which Element Is Found In All Organic Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
