Coordination Number Of Body Centered Cubic
News Leon
Mar 14, 2025 · 5 min read
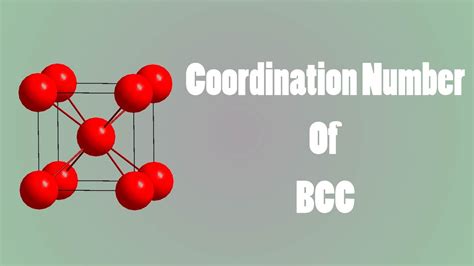
Table of Contents
Coordination Number of Body-Centered Cubic (BCC) Structure: A Deep Dive
The coordination number in crystallography represents the number of nearest neighbors surrounding a central atom in a crystal lattice. Understanding coordination numbers is crucial for predicting material properties, such as density, melting point, and mechanical strength. This article delves into the coordination number of the body-centered cubic (BCC) structure, explaining its calculation, significance, and implications for various materials exhibiting this structure.
Understanding the Body-Centered Cubic (BCC) Structure
The body-centered cubic (BCC) structure is one of the most common crystal structures found in metals and alloys. It's characterized by a cubic unit cell containing:
- One atom at each of the eight corners of the cube. Each corner atom is shared by eight adjacent unit cells, contributing 1/8 of an atom to the unit cell.
- One atom located at the center of the cube. This atom is entirely within the unit cell and contributes one full atom.
Therefore, the total number of atoms per unit cell in a BCC structure is: (8 corner atoms × 1/8 atom/corner) + 1 center atom = 2 atoms.
Calculating the Coordination Number in BCC
Determining the coordination number involves identifying the nearest neighboring atoms surrounding a central atom. In the BCC structure:
-
Consider a central atom: Let's focus on the atom located at the center of the unit cell.
-
Identify nearest neighbors: The nearest neighbors to this central atom are the eight corner atoms. They are equidistant from the central atom.
-
Determine the coordination number: Since there are eight nearest neighbors, the coordination number of the central atom in a BCC structure is 8.
It's important to note that this applies to every atom within a perfect BCC lattice. Each atom, whether at the corner or the center, has eight equidistant nearest neighbors. This uniformity is a defining characteristic of the BCC structure.
The Significance of Coordination Number 8 in BCC
The coordination number of 8 in BCC structures has profound implications for the properties of materials exhibiting this arrangement.
1. Atomic Packing Efficiency (APE)
The atomic packing efficiency represents the fraction of space within a unit cell occupied by atoms. In BCC structures, the APE is approximately 68%. This relatively lower APE compared to face-centered cubic (FCC) structures (74%) influences several material properties.
2. Density
The lower APE directly contributes to a lower density in BCC metals compared to their FCC counterparts, assuming similar atomic weights. This difference in density can be observed in many metals that can exist in both BCC and FCC structures under different conditions.
3. Mechanical Properties
The coordination number and resulting atomic arrangement influence the mechanical properties of BCC materials. The relatively less tightly packed structure contributes to:
- Higher ductility: BCC metals tend to exhibit greater ductility at higher temperatures, meaning they can undergo significant deformation before fracture. However, at lower temperatures, they can become brittle.
- Moderate Strength: BCC materials generally show moderate tensile strength compared to other crystal structures.
- Anisotropy: BCC metals often exhibit anisotropy, meaning their properties vary depending on the direction. This is due to the directional nature of the bonding between atoms in the structure.
4. Thermal Properties
The coordination number also subtly influences thermal properties. The less efficient packing can affect thermal conductivity and thermal expansion coefficients.
BCC Structure in Different Materials
Numerous elements and alloys exhibit the BCC structure, each exhibiting unique properties based on their specific atomic arrangements and interactions.
Metals
Many transition metals, including:
- Chromium (Cr)
- Molybdenum (Mo)
- Tungsten (W)
- Vanadium (V)
- Iron (Fe) (at room temperature)
- Niobium (Nb)
- Tantalum (Ta)
exhibit a BCC structure at room temperature. Their properties, such as high melting points and high strength, are linked to their strong metallic bonding and BCC crystal structure.
Alloys
Many alloys also adopt the BCC structure. The addition of alloying elements can significantly modify the properties of BCC metals by influencing factors like grain size, dislocation density, and solid solution strengthening.
Intermetallic Compounds
Some intermetallic compounds also crystallize in BCC structures. These compounds usually have unique properties that are different from those of the constituent elements.
Comparison with Other Crystal Structures
Comparing the BCC structure to other common crystal structures, like face-centered cubic (FCC) and hexagonal close-packed (HCP), highlights the significance of coordination number:
| Structure | Coordination Number | Atomic Packing Efficiency | Typical Properties |
|---|---|---|---|
| Body-Centered Cubic (BCC) | 8 | ~68% | Moderate strength, higher ductility at high temps |
| Face-Centered Cubic (FCC) | 12 | ~74% | High ductility, high strength |
| Hexagonal Close-Packed (HCP) | 12 | ~74% | High strength, lower ductility |
The difference in coordination number directly influences the atomic packing efficiency, leading to variations in material properties. FCC and HCP structures, with their higher coordination numbers, possess greater atomic packing density and, consequently, different mechanical and physical characteristics compared to BCC.
Advanced Concepts and Applications
The coordination number in BCC structures is a foundational concept with implications for more advanced topics:
- Defect Analysis: Understanding the coordination number helps analyze crystal defects like vacancies, interstitials, and dislocations within the BCC lattice. These defects significantly influence material properties.
- Phase Transformations: Many BCC metals undergo phase transformations, changing their crystal structure under varying conditions of temperature and pressure. The coordination number plays a critical role in understanding these transitions.
- Computational Materials Science: Simulations and modeling of BCC materials often rely heavily on the coordination number to accurately predict material behaviors and properties.
- Alloy Design: Knowledge of the coordination number is crucial in alloy design and development, allowing scientists to tailor materials with specific desired properties.
Conclusion
The coordination number of 8 in the body-centered cubic (BCC) structure is a fundamental characteristic defining the arrangement of atoms and profoundly impacting the material's properties. This number dictates the atomic packing efficiency, influencing density, mechanical strength, ductility, and other crucial attributes. Understanding the coordination number in BCC structures is essential for comprehending the behavior and applications of numerous metals and alloys in diverse engineering and technological domains. Further research into this area continues to reveal more intricate details about the relationship between atomic arrangements and macroscopic material properties, providing valuable insights for materials science and engineering.
Latest Posts
Latest Posts
-
9 Is What Percent Of 72
Mar 15, 2025
-
How Many Valence Electrons Are In H
Mar 15, 2025
-
A Regular Quadrilateral Has What Type Of Symmetry
Mar 15, 2025
-
What Is The Conjugate Acid Of Oh
Mar 15, 2025
-
Which Of The Following Is Not A Renewable Resource
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Coordination Number Of Body Centered Cubic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
