What Is The Conjugate Acid Of Oh
News Leon
Mar 15, 2025 · 6 min read
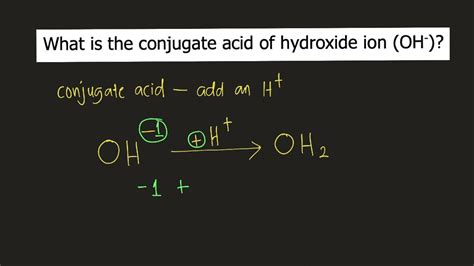
Table of Contents
What is the Conjugate Acid of OH⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article will delve into the concept, focusing specifically on the conjugate acid of the hydroxide ion (OH⁻). We'll explore the definition of conjugate acid-base pairs, the Brønsted-Lowry theory, and the implications of understanding this relationship in various chemical contexts.
Understanding Conjugate Acid-Base Pairs
According to the Brønsted-Lowry theory, an acid is a proton (H⁺) donor, and a base is a proton acceptor. A conjugate acid-base pair consists of two species that differ by a single proton (H⁺). When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. This relationship is crucial in understanding acid-base reactions and equilibrium.
The Role of Protons in Conjugate Pairs
The key to identifying conjugate pairs lies in the transfer of a single proton. Let's illustrate this with a simple example: the reaction between hydrochloric acid (HCl) and water (H₂O).
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
In this reaction:
- HCl acts as an acid, donating a proton to water.
- H₂O acts as a base, accepting a proton from HCl.
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O.
- Cl⁻ (chloride ion) is the conjugate base of HCl.
Notice that H₃O⁺ and H₂O differ by a single proton, as do HCl and Cl⁻. This difference defines the conjugate acid-base relationship.
Identifying the Conjugate Acid of OH⁻
Now, let's apply this understanding to the hydroxide ion (OH⁻). The hydroxide ion is a strong base; it readily accepts a proton. To find its conjugate acid, we simply add a proton (H⁺):
OH⁻ + H⁺ → H₂O
Therefore, the conjugate acid of OH⁻ is H₂O (water).
This seemingly simple answer reveals a profound implication: water can act as both an acid and a base. This amphoteric nature of water is crucial in many chemical processes.
The Amphoteric Nature of Water and its Significance
Water's ability to act as both an acid and a base is a direct consequence of its conjugate acid-base relationship with OH⁻ and H₃O⁺. This amphoteric behavior is evident in the autoionization of water:
2H₂O ⇌ H₃O⁺ + OH⁻
In this equilibrium reaction:
- One water molecule acts as an acid, donating a proton.
- The other water molecule acts as a base, accepting a proton.
This equilibrium constantly exists in aqueous solutions, determining the pH of pure water and influencing the behavior of acids and bases dissolved in it. The equilibrium constant for this reaction (Kw) is crucial in calculating pH and pOH.
Implications in Acid-Base Titrations
Understanding the conjugate acid-base relationship, particularly for water and OH⁻, is vital in acid-base titrations. Titrations involve the gradual addition of an acid or base to a solution of the opposite type, monitoring the pH change to determine the equivalence point. The behavior of water and its conjugate ions significantly impacts the pH changes during a titration, especially near the equivalence point.
Buffer Solutions and Conjugate Pairs
Buffer solutions are crucial in maintaining a relatively constant pH despite the addition of small amounts of acid or base. These solutions typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). The conjugate acid-base pair in a buffer solution works to neutralize added H⁺ or OH⁻ ions, minimizing pH fluctuations.
The Importance of Conjugate Acid-Base Pairs in Biological Systems
The concept of conjugate acid-base pairs is immensely important in biological systems. Many biochemical reactions occur in aqueous solutions, and the behavior of water and its conjugate ions plays a critical role in maintaining the appropriate pH for these reactions to proceed effectively. Proteins, enzymes, and DNA all depend on the careful regulation of pH, which is directly linked to the equilibrium between H₃O⁺ and OH⁻.
Beyond the Basics: Exploring Different Acid-Base Theories
While the Brønsted-Lowry theory provides a robust framework for understanding conjugate acid-base pairs, other theories exist, offering alternative perspectives.
The Lewis Theory of Acids and Bases
The Lewis theory expands the definition of acids and bases beyond proton transfer. A Lewis acid is defined as an electron-pair acceptor, while a Lewis base is an electron-pair donor. While the Brønsted-Lowry definition is sufficient for many scenarios, the Lewis theory can explain reactions that don't involve proton transfer. For instance, the reaction between boron trifluoride (BF₃) and ammonia (NH₃) is considered an acid-base reaction according to the Lewis theory, but not according to the Brønsted-Lowry theory.
Applying the Lewis Theory to OH⁻
Applying the Lewis theory to the hydroxide ion, we see that it acts as a Lewis base because it has a lone pair of electrons that it can donate. When it accepts a proton (H⁺), it forms water (H₂O), its conjugate acid. The proton in this case acts as a Lewis acid, accepting the electron pair from the hydroxide ion.
Practical Applications and Further Exploration
Understanding the conjugate acid of OH⁻ and the broader concept of conjugate acid-base pairs has wide-ranging applications across various fields, including:
- Analytical Chemistry: In quantitative analysis, acid-base titrations rely heavily on this concept. Understanding buffer solutions and the behavior of conjugate acid-base pairs is crucial for accurate results.
- Environmental Science: Acid rain and water quality assessments require understanding the interactions between different acids and bases in water systems. The concept of pH and its relationship to conjugate acid-base pairs is central to these assessments.
- Biochemistry and Medicine: The pH of body fluids is tightly regulated, and deviations can have serious health consequences. Buffers play a crucial role in maintaining this pH balance, making understanding conjugate acid-base pairs vital for medical professionals and researchers.
- Material Science: The synthesis and properties of many materials are affected by pH and acid-base reactions. Understanding the role of conjugate acid-base pairs is crucial for the development of new materials with specific properties.
Conclusion
The conjugate acid of OH⁻ is water (H₂O). This simple fact underpins a deep understanding of acid-base chemistry, encompassing the Brønsted-Lowry theory, the amphoteric nature of water, and the importance of conjugate pairs in various chemical and biological systems. By understanding the fundamental principles of conjugate acid-base pairs, we can gain a more profound appreciation for the intricacies of acid-base reactions and their impact on numerous fields of study and applications. Further exploration of acid-base theories, such as the Lewis theory, enriches this understanding and expands the scope of our knowledge.
Latest Posts
Latest Posts
-
Friction Is Always Opposite To The Direction Of Motion
Mar 15, 2025
-
What Is The Difference Between An Enzyme And A Hormone
Mar 15, 2025
-
What Are Two Main Categories Of Software
Mar 15, 2025
-
What Is The Closest Planet To The Moon
Mar 15, 2025
-
A Lever Rotates Around A Fixed Point Called A
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid Of Oh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
