How Many Valence Electrons Are In H
News Leon
Mar 15, 2025 · 5 min read
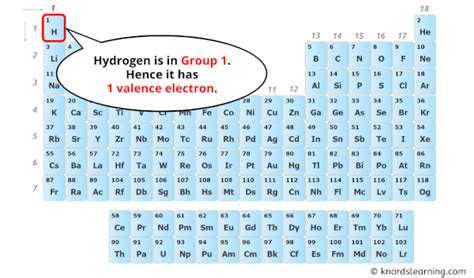
Table of Contents
How Many Valence Electrons Are in Hydrogen? A Deep Dive into Atomic Structure
Hydrogen, the simplest element in the periodic table, holds a unique position in chemistry. Understanding its atomic structure, particularly the number of valence electrons it possesses, is crucial to grasping its reactivity and role in various chemical processes. This comprehensive guide will delve into the specifics of hydrogen's valence electrons, exploring its electronic configuration, bonding behavior, and significance in different contexts.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we specifically address hydrogen, let's define what valence electrons are. Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the most loosely bound to the nucleus and are therefore most likely to participate in chemical bonding. The number of valence electrons an atom possesses determines its reactivity and the types of bonds it can form. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outermost shell – a concept known as the octet rule (though this rule has exceptions, particularly with elements like hydrogen).
Hydrogen's Electronic Configuration: A Singular Electron
Hydrogen (H), with an atomic number of 1, possesses only one proton in its nucleus and, in its neutral state, one electron. This single electron occupies the atom's first and only electron shell, specifically the 1s orbital. The 1s orbital can hold a maximum of two electrons. Therefore, hydrogen's electronic configuration is written as 1s<sup>1</sup>.
The Significance of the 1s Orbital
The 1s orbital is a spherical region of space surrounding the nucleus where there's a high probability of finding the electron. Because it's the closest orbital to the nucleus, the electron in the 1s orbital is relatively strongly bound. This strong binding influences hydrogen's chemical behavior.
Determining the Number of Valence Electrons in Hydrogen
Since hydrogen's sole electron resides in its outermost shell (the 1s orbital is the only shell in this case), it has one valence electron. This single valence electron is the key to understanding hydrogen's remarkable reactivity and its ability to form various chemical bonds.
Hydrogen's Bonding Behavior: A Versatile Element
Hydrogen's single valence electron allows it to participate in several types of chemical bonding:
1. Covalent Bonding: Sharing is Caring
Hydrogen frequently participates in covalent bonding, where it shares its single valence electron with another atom to achieve a more stable electron configuration. A classic example is the hydrogen molecule (H<sub>2</sub>), where two hydrogen atoms share their electrons, forming a single covalent bond. Each hydrogen atom effectively attains a filled 1s orbital, resembling the stable electron configuration of helium.
2. Ionic Bonding: Giving and Taking
While less common, hydrogen can also participate in ionic bonding, particularly when bonding with highly electronegative atoms like halogens (e.g., fluorine, chlorine). In these instances, hydrogen can lose its electron to become a positively charged hydrogen ion (H<sup>+</sup>), often referred to as a proton. This type of bonding is characteristic of hydrogen halides such as hydrogen fluoride (HF) and hydrogen chloride (HCl).
3. Metallic Bonding: A Less Familiar Role
In certain metallic hydrides, hydrogen can also participate in metallic bonding, where valence electrons are delocalized across a lattice of metal atoms. However, this type of bonding is less prevalent compared to covalent and ionic bonding for hydrogen.
Hydrogen's Unique Properties: A Consequence of its Single Valence Electron
Hydrogen's single valence electron is responsible for many of its unique properties:
- Low Density: Hydrogen is the lightest element, with a very low density due to its small atomic mass.
- High Reactivity (in some cases): The single valence electron makes it readily available for bonding, although the strength of this reactivity depends on the partner atom.
- Versatile Bonding Capabilities: As discussed above, hydrogen can form various types of bonds.
- Abundance: Hydrogen is the most abundant element in the universe.
The Importance of Hydrogen in Chemistry and Beyond
Hydrogen's unique properties and simple structure have made it a pivotal element in numerous fields:
1. Industrial Applications: Fuel and More
Hydrogen is increasingly used as a fuel source, especially in fuel cells that produce electricity through the electrochemical reaction of hydrogen and oxygen. It's also utilized in the production of ammonia (a crucial component of fertilizers) through the Haber-Bosch process. Hydrogen is also applied in petroleum refining and metallurgy.
2. Biological Significance: Essential for Life
Hydrogen plays a vital role in biological systems. It’s a fundamental component of water (H<sub>2</sub>O), which is essential for all living organisms. It also participates in numerous biological molecules like carbohydrates, proteins, and lipids.
3. Emerging Technologies: A Future Fuel?
With growing concerns about climate change and the need for cleaner energy sources, hydrogen is being explored as a potential future fuel. Research is underway to develop efficient and cost-effective methods for hydrogen production, storage, and transportation.
Hydrogen Isotopes and Valence Electrons: Deuterium and Tritium
It's important to note that hydrogen has three isotopes: protium (<sup>1</sup>H), deuterium (<sup>2</sup>H or D), and tritium (<sup>3</sup>H or T). These isotopes differ in the number of neutrons in their nuclei but share the same number of protons and electrons. Consequently, all isotopes of hydrogen have one valence electron. The differences in neutron number affect their mass and some nuclear properties but not their valence electron count or fundamental chemical reactivity.
Conclusion: A Single Electron with Profound Implications
Hydrogen's single valence electron is the key to understanding its remarkable chemical behavior and wide-ranging applications. Its ability to form various bonds, its low density, and its abundance make it a crucial element in chemistry, biology, and emerging technologies. While seemingly simple, hydrogen's one valence electron has far-reaching consequences, making it an element worthy of continued study and appreciation. Further research into hydrogen's properties and applications will undoubtedly unveil even more of its vital contributions to our world.
Latest Posts
Latest Posts
-
What Is The Distance From The Sun For Saturn
Mar 15, 2025
-
Prove Square Root 3 Is Irrational
Mar 15, 2025
-
Which Of The Following Is An Example Of A Suspension
Mar 15, 2025
-
Which Country Is Called The Land Of Rising Sun
Mar 15, 2025
-
Is Mercury A Heavier Element Than Tin
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In H . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
