Which Of The Following Is An Example Of A Suspension
News Leon
Mar 15, 2025 · 6 min read
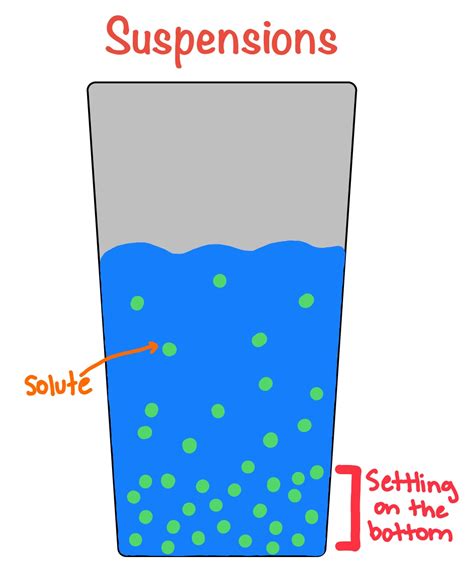
Table of Contents
Which of the Following is an Example of a Suspension? Understanding Colloids and Their Properties
Understanding the difference between solutions, colloids, and suspensions is crucial in many scientific fields, from chemistry and biology to environmental science and materials engineering. This article delves deep into the properties of suspensions, providing clear examples and differentiating them from solutions and colloids. We’ll explore the characteristics that define a suspension and examine various everyday examples to solidify your understanding. By the end, you'll confidently be able to identify a suspension from a list of options.
What is a Suspension?
A suspension is a heterogeneous mixture where solid particles are dispersed throughout a liquid. The key difference between a suspension and other mixtures lies in the size of the dispersed particles. In a suspension, these particles are relatively large, typically greater than 1000 nanometers (1 micrometer) in diameter. This large particle size is what leads to several key characteristics:
- Heterogeneous nature: Unlike a solution, where components are uniformly distributed at a molecular level, suspensions are visibly heterogeneous. You can easily distinguish the solid particles from the liquid medium.
- Settling: Over time, the larger particles in a suspension will settle out of the liquid due to gravity. This is a hallmark characteristic of suspensions.
- Easily separated: The solid particles in a suspension can be easily separated from the liquid by methods such as filtration or sedimentation.
- Cloudy or opaque appearance: The presence of large particles usually results in a cloudy or opaque appearance. Light is scattered by these particles, preventing it from passing through easily.
Suspensions vs. Solutions vs. Colloids: Key Differences
To fully grasp what constitutes a suspension, it's essential to contrast it with solutions and colloids. Here's a comparative table highlighting their differences:
| Feature | Solution | Colloid | Suspension |
|---|---|---|---|
| Particle Size | < 1 nm | 1-1000 nm | > 1000 nm |
| Appearance | Transparent | Translucent or opaque | Opaque |
| Settling | No settling | No settling (usually) | Settling occurs |
| Filtration | Particles pass through | Particles pass through | Particles do not pass through |
| Heterogeneity | Homogeneous | Heterogeneous (but appears homogeneous) | Heterogeneous |
| Examples | Saltwater, sugar water | Milk, fog, paint | Muddy water, sand in water |
Examples of Suspensions in Everyday Life
Numerous examples of suspensions are readily observable in our daily lives. Here are some common instances:
1. Muddy Water: A Classic Suspension
Muddy water is perhaps the most straightforward example of a suspension. The soil particles, including clay, silt, and sand, are suspended in the water. If left undisturbed, these particles will gradually settle to the bottom of the container. You can easily filter out the solid particles from the water.
2. Flour in Water: A Simple Kitchen Suspension
Mixing flour in water creates a suspension. The flour particles are initially dispersed, giving the mixture a cloudy appearance. However, given enough time, the flour particles will settle to the bottom. This is a simple demonstration that highlights the characteristic settling behavior of suspensions.
3. Sand in Water: Another Easily Observable Suspension
Similar to muddy water, a mixture of sand and water represents a classic suspension. The larger sand particles are clearly visible and will quickly settle out when the mixture is left standing. This simple experiment helps visually understand the heterogeneous nature of a suspension.
4. Pharmaceutical Suspensions: Oral Medications
Many liquid oral medications are formulated as suspensions. The active pharmaceutical ingredient is finely dispersed in a liquid carrier. These suspensions need to be shaken well before administration to ensure even distribution of the medication. The settling of the active ingredient over time is a key factor in their formulation and instructions for use.
5. Paint: A Suspension of Pigments in a Liquid
Paint is a suspension of pigment particles (the color) in a liquid binder (like oil or water). The pigment particles are relatively large and contribute to the opaque nature of paint. They remain suspended due to the properties of the binder, but some settling can still occur over time. Shaking the can before application is necessary to redistribute the pigment particles for a uniform coating.
6. Calamine Lotion: A Medicated Suspension
Calamine lotion, a common topical medication for skin irritations, is another example of a suspension. The active ingredients are dispersed in a liquid base, forming a suspension that needs to be shaken before use to ensure even application.
7. Tomato Ketchup: A Complex Suspension
Though it may seem homogenous initially, tomato ketchup is actually a complex suspension. The solid particles of spices, pulp, and other ingredients are dispersed in a liquid matrix. A certain level of settling may occur depending on the concentration and additives, showcasing its suspension-like properties.
Applications of Suspensions
Suspensions find extensive use in various industries and applications:
- Pharmaceuticals: As previously mentioned, many oral and topical medications are formulated as suspensions for controlled drug delivery.
- Cosmetics: Several cosmetic products, such as lotions, creams, and sunscreens, are suspensions of active ingredients and pigments in a suitable base.
- Paints and Coatings: Suspensions are the basis for various paints, coatings, and inks used in construction, automotive, and other industries.
- Agriculture: Certain pesticides and fertilizers are formulated as suspensions for better application and distribution.
- Food Industry: Several food products, including some sauces, dressings, and beverages, utilize suspension properties in their formulation.
- Environmental Remediation: Suspensions are involved in processes related to water treatment and environmental cleanup.
Factors Affecting Suspension Stability
The stability of a suspension—its ability to remain suspended without settling—depends on several factors:
- Particle Size: Smaller particles tend to remain suspended longer due to reduced sedimentation rate.
- Particle Density: Particles with a density similar to the liquid phase will remain suspended longer.
- Viscosity of the Liquid: A higher viscosity liquid hinders sedimentation, improving suspension stability.
- Presence of Stabilizers: Additives such as dispersing agents or thickening agents can increase suspension stability by preventing particle aggregation and settling.
Identifying Suspensions: A Practical Approach
When trying to identify a suspension from a list of options, focus on the following characteristics:
- Visual inspection: Check the appearance. Is the mixture cloudy or opaque?
- Settling test: Allow the mixture to settle. Do the solid particles separate from the liquid over time?
- Filtration test: Try filtering the mixture. Do the solid particles get trapped on the filter paper?
If the mixture displays a cloudy/opaque appearance, settles upon standing, and its solid particles are retained upon filtration, it is likely a suspension.
Conclusion: Understanding the Nuances of Suspensions
Suspensions are ubiquitous in everyday life and critical to various industries. Understanding their properties—heterogeneous nature, settling behavior, and the ability to separate components by filtration—is vital. By recognizing the key differences between suspensions, solutions, and colloids, you can accurately identify suspensions and appreciate their wide-ranging applications. This understanding extends beyond simple identification to comprehending the intricate scientific principles underlying their behavior and stability. Remember to consider factors like particle size, density, and viscosity when analyzing the stability of a particular suspension. This comprehensive knowledge empowers you to navigate various scenarios, from identifying household substances to understanding complex industrial processes involving suspensions.
Latest Posts
Latest Posts
-
What Is The Oxidation State Of Mn In Kmno4
Mar 15, 2025
-
Astronauts On The International Space Station Are Weightless Because
Mar 15, 2025
-
Compressibility In Solids Liquids And Gases
Mar 15, 2025
-
What Is 75 Celsius In Fahrenheit
Mar 15, 2025
-
What Is The Molar Mass Of Oxygen O2
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Example Of A Suspension . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
