What Is The Oxidation State Of Mn In Kmno4
News Leon
Mar 15, 2025 · 5 min read
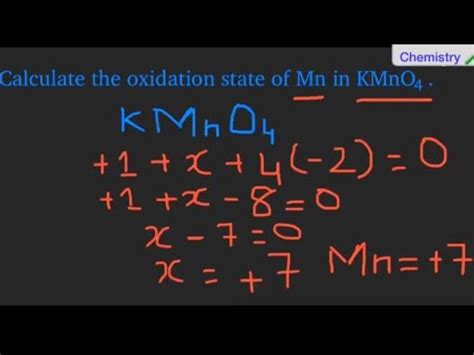
Table of Contents
What is the Oxidation State of Mn in KMnO₄? A Deep Dive into Oxidation Numbers
Determining the oxidation state of manganese (Mn) in potassium permanganate (KMnO₄) is a fundamental concept in chemistry, crucial for understanding its redox reactions and applications. This comprehensive guide will delve into the process of calculating the oxidation state, exploring the underlying principles and providing a detailed explanation. We'll also examine the implications of this oxidation state for KMnO₄'s properties and its role in various chemical reactions.
Understanding Oxidation States
Before we tackle the specific case of KMnO₄, let's establish a clear understanding of what oxidation states, also known as oxidation numbers, represent. An oxidation state is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. It's a useful tool for tracking electron transfer during chemical reactions, particularly redox (reduction-oxidation) reactions.
While not a true charge, the oxidation state helps us predict the reactivity and behavior of elements and compounds. It's essential to remember that the oxidation state is a formal assignment; it doesn't represent the actual charge on an atom, especially in covalent compounds where electrons are shared, not fully transferred.
Rules for Assigning Oxidation States
Assigning oxidation states follows a set of rules, prioritized in the following order:
-
Free elements: The oxidation state of an atom in its elemental form is always 0 (e.g., O₂ has an oxidation state of 0 for each oxygen atom).
-
Monatomic ions: The oxidation state of a monatomic ion is equal to its charge (e.g., Na⁺ has an oxidation state of +1, Cl⁻ has an oxidation state of -1).
-
Hydrogen: Hydrogen typically has an oxidation state of +1, except in metal hydrides where it is -1 (e.g., NaH).
-
Oxygen: Oxygen usually has an oxidation state of -2, except in peroxides (like H₂O₂) where it's -1 and in superoxides where it's -1/2.
-
Group 1 and 2 elements: Group 1 elements (alkali metals) always have an oxidation state of +1, and Group 2 elements (alkaline earth metals) always have an oxidation state of +2.
-
The sum of oxidation states: In a neutral compound, the sum of the oxidation states of all atoms must equal zero. In a polyatomic ion, the sum of the oxidation states must equal the charge of the ion.
Calculating the Oxidation State of Mn in KMnO₄
Now, let's apply these rules to determine the oxidation state of Mn in KMnO₄:
- Potassium (K): Potassium is an alkali metal (Group 1), so its oxidation state is +1.
- Oxygen (O): Oxygen typically has an oxidation state of -2. Since there are four oxygen atoms in KMnO₄, the total contribution from oxygen is 4 * (-2) = -8.
Let's represent the oxidation state of manganese as 'x'. Since KMnO₄ is a neutral compound, the sum of the oxidation states must be zero:
(+1) + x + (-8) = 0
Solving for x:
x = +7
Therefore, the oxidation state of Mn in KMnO₄ is +7.
Significance of the +7 Oxidation State of Mn in KMnO₄
The +7 oxidation state of manganese in KMnO₄ is highly significant, contributing to its strong oxidizing properties. This high oxidation state means that manganese is readily reduced, gaining electrons and causing the oxidation of other species. This makes KMnO₄ a powerful oxidizing agent, widely used in various chemical reactions and applications.
Applications of KMnO₄ as a Strong Oxidizing Agent:
-
Titrations: KMnO₄ is a common titrant in redox titrations due to its intense purple color, which disappears as it is reduced. The endpoint of the titration is easily observed, making it a valuable tool in quantitative analysis.
-
Water Treatment: KMnO₄ is used to disinfect water by oxidizing harmful bacteria and other microorganisms. Its oxidizing power eliminates pathogens, improving water quality and safety.
-
Organic Synthesis: In organic chemistry, KMnO₄ is utilized as an oxidant in various reactions, including the oxidation of alcohols to aldehydes or ketones, and the cleavage of carbon-carbon double bonds.
-
Medicine: KMnO₄ has antiseptic properties and is sometimes used as a disinfectant for minor wounds. However, its use in medicine is limited due to potential toxicity.
-
Analytical Chemistry: The ability of KMnO₄ to oxidize various substances allows for its use in analytical chemistry for determining the concentration of reducing agents in samples.
Redox Reactions involving KMnO₄
The high oxidation state of manganese in KMnO₄ makes it a versatile participant in redox reactions. The reduction of Mn(VII) to lower oxidation states, such as Mn(II), Mn(IV), or Mn(III), drives many of its applications. The specific reduction product depends on the reaction conditions, such as pH and the nature of the reducing agent.
Examples of Redox Reactions:
-
Reaction with Oxalic Acid: In acidic solution, KMnO₄ oxidizes oxalic acid (C₂H₂O₄), itself being reduced to Mn²⁺ (Mn(II)). The reaction is self-indicating, as the purple colour of KMnO₄ fades as the reaction proceeds.
-
Reaction with Ferrous Ions: In acidic solution, KMnO₄ oxidizes ferrous ions (Fe²⁺) to ferric ions (Fe³⁺), while Mn(VII) is reduced to Mn²⁺. This reaction is widely used in titrimetric analysis.
Understanding the Electronic Configuration
The +7 oxidation state of manganese in KMnO₄ can also be understood through its electronic configuration. Manganese's ground state electronic configuration is [Ar] 3d⁵ 4s². In the +7 oxidation state, it loses all seven of its outermost electrons (five 3d electrons and two 4s electrons), achieving a stable electronic configuration, although not a noble gas configuration.
Safety Precautions when Handling KMnO₄
Potassium permanganate is a powerful oxidizing agent and should be handled with care. It can cause skin irritation and staining, and its solutions should be kept away from flammable materials. Always wear appropriate protective equipment, including gloves and eye protection, when handling KMnO₄.
Conclusion
The determination of the oxidation state of Mn in KMnO₄ as +7 is a critical step in understanding its chemical behavior and wide-ranging applications. Its strong oxidizing properties, stemming from this high oxidation state, make it a valuable reagent in diverse fields, from analytical chemistry and water treatment to organic synthesis. However, safe handling practices are essential due to its potent oxidizing nature. By understanding the fundamental principles of oxidation states and the specific properties of KMnO₄, we can effectively harness its capabilities while mitigating potential risks. This comprehensive analysis provides a robust foundation for further exploration of this crucial compound in various chemical contexts.
Latest Posts
Latest Posts
-
If A Pea Plant Shows A Recessive Phenotype
Mar 15, 2025
-
Is A Patent A Current Asset
Mar 15, 2025
-
Why Are Producers So Important To An Ecosystem
Mar 15, 2025
-
Difference Between Interest Groups And Political Parties
Mar 15, 2025
-
The Division Of The Cell Nucleus Is Called
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of Mn In Kmno4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
