Cells Placed In A Hypertonic Solution Will
News Leon
Mar 14, 2025 · 5 min read
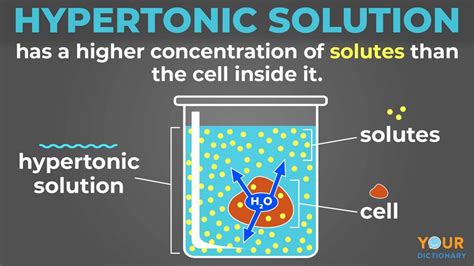
Table of Contents
Cells Placed in a Hypertonic Solution Will: A Deep Dive into Osmosis and Cell Behavior
Understanding how cells react to different environments is fundamental to biology. One crucial concept is osmosis, the movement of water across a selectively permeable membrane. When a cell is placed in a hypertonic solution, a fascinating and often dramatic process unfolds. This article will explore what happens to cells in a hypertonic solution, delving into the mechanisms involved, the consequences for different cell types, and the broader implications of this phenomenon in biology and medicine.
What is a Hypertonic Solution?
Before we dive into the effects, let's define our terms. A hypertonic solution is one with a higher solute concentration compared to another solution. This "other solution" is typically the cytoplasm inside a cell. The solute concentration refers to the amount of dissolved substances (like salts, sugars, or proteins) present in the solution. Water, being the solvent, moves to equalize the concentration across the membrane.
Think of it like this: imagine a party where there are many more people (solutes) outside a room (cell) than inside. Naturally, people (water molecules) will tend to move from the less crowded area (inside the cell) to the more crowded area (outside the cell) to try and even things out.
Osmosis: The Driving Force
Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration). This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. This process is driven by the difference in water potential between the two solutions.
The selectively permeable membrane is crucial. It allows water molecules to pass through but restricts the passage of most solutes. This selective permeability is what makes osmosis a unique and vital process for cells.
What Happens to Cells in a Hypertonic Solution?
When a cell is placed in a hypertonic solution, the water concentration is higher inside the cell than outside. Consequently, water will move out of the cell across the cell membrane into the surrounding hypertonic solution via osmosis. This outward movement of water causes the cell to shrink or crenate.
The extent of crenation depends on several factors, including:
- The initial cell turgor pressure: Cells that are initially turgid (firm) will lose more water and shrink more dramatically than cells that are already somewhat flaccid.
- The concentration gradient: A steeper concentration gradient (a larger difference in solute concentration between the inside and outside of the cell) will lead to a faster rate of water loss and more pronounced crenation.
- The cell wall (if present): Plant cells and some bacterial cells have a rigid cell wall surrounding the cell membrane. This wall provides structural support and prevents excessive shrinking. However, even in plant cells, prolonged exposure to a hypertonic solution can lead to plasmolysis, where the cell membrane pulls away from the cell wall.
Animal Cells in Hypertonic Solutions
Animal cells, lacking a rigid cell wall, are particularly vulnerable to hypertonic environments. The continuous loss of water leads to significant shrinkage and potential damage to cellular structures. Severe crenation can disrupt cellular processes and ultimately lead to cell death.
Consequences for animal cells in hypertonic solutions:
- Cell shrinkage: The most immediate and visible effect.
- Disruption of cellular processes: Water loss can affect the proper functioning of enzymes and other cellular components.
- Damage to cell membranes and organelles: Extreme shrinkage can lead to damage or rupture of the cell membrane and organelles.
- Cell death (lysis): In extreme cases, the cell may die due to the severe disruption of its internal environment.
Plant Cells in Hypertonic Solutions
Plant cells, with their cell walls, respond differently. While water loss still occurs, the cell wall prevents complete collapse. Instead, the cell membrane pulls away from the cell wall, a process called plasmolysis.
Consequences for plant cells in hypertonic solutions:
- Plasmolysis: The cell membrane detaches from the cell wall.
- Loss of turgor pressure: The cell loses its firmness and becomes flaccid.
- Reduced metabolic activity: The reduced turgor pressure can affect the transport of nutrients and the overall metabolic activity of the plant cell.
- Wilting (in whole plants): When many plant cells undergo plasmolysis, the plant as a whole wilts.
Bacterial Cells in Hypertonic Solutions
Bacterial cells also possess a cell wall, but their response to hypertonic stress can vary depending on the species and the specific conditions. Similar to plant cells, plasmolysis can occur, but the cell wall structure can influence the severity of the effect. Some bacteria have mechanisms to adapt to high-solute environments, accumulating compatible solutes to balance osmotic pressure.
Practical Applications and Importance
Understanding the effects of hypertonic solutions has significant implications across various fields:
- Food preservation: High salt or sugar concentrations in preserved foods create hypertonic environments that inhibit microbial growth by causing plasmolysis in microorganisms.
- Medicine: Intravenous solutions must be carefully balanced to be isotonic (same solute concentration as blood) to prevent damaging effects on red blood cells.
- Agriculture: Understanding osmosis helps farmers manage soil salinity and irrigation practices to prevent plant damage caused by hypertonic soil solutions.
- Cellular biology research: Studying cellular responses to hypertonic conditions provides insights into cell membrane function, osmotic regulation, and stress responses.
Beyond Osmosis: Other Factors
While osmosis is the primary driving force, other factors influence the overall cell response in hypertonic solutions:
- Cell type: Different cell types have varying tolerances to osmotic stress.
- Solute type: The specific type of solute in the hypertonic solution can influence the cellular response, some solutes being more toxic than others.
- Duration of exposure: Prolonged exposure to a hypertonic solution generally leads to more severe effects.
Conclusion
The effects of placing cells in a hypertonic solution are a complex interplay of osmotic pressure, cell wall structure (if present), and cellular mechanisms. Understanding these effects is critical in various biological and practical applications. From preserving food to improving agricultural practices and advancing medical treatments, the principles of osmosis and hypertonic solutions are deeply intertwined with many facets of our lives. The ability of cells to withstand, adapt, or succumb to hypertonic environments reflects the fundamental balance between survival and environmental pressures inherent in biological systems. Further research into these cellular responses will continue to unlock new possibilities for a deeper comprehension of the intricacies of life at the cellular level.
Latest Posts
Latest Posts
-
Which Chamber Of The Heart Has The Thickest Muscular Wall
Mar 14, 2025
-
The Product Of Two Irrational Numbers Is Irrational
Mar 14, 2025
-
Which Element Is Found In All Organic Compounds
Mar 14, 2025
-
What Is The Largest Single Mass Of Lymphatic Tissue
Mar 14, 2025
-
How Many Radians Is One Revolution
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Cells Placed In A Hypertonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
