How Many Valence Electrons Does Cr Have
News Leon
Mar 19, 2025 · 6 min read
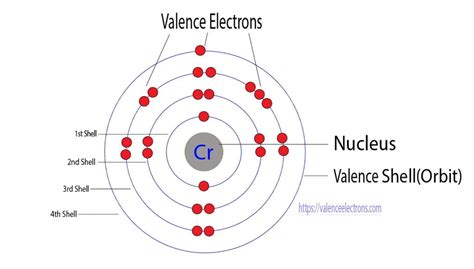
Table of Contents
How Many Valence Electrons Does Chromium (Cr) Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Chromium (Cr), a lustrous, hard, and brittle transition metal, finds itself at the heart of numerous applications, from stainless steel to pigments. Understanding its chemical behavior hinges on comprehending its electronic structure, specifically the number of valence electrons it possesses. This article delves deep into the electronic configuration of chromium, explaining why it deviates from the expected pattern and exploring the implications of this anomaly on its chemical properties and reactivity.
Understanding Valence Electrons: The Key to Chemical Bonding
Before focusing on chromium, let's briefly review the concept of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are crucial because they participate in chemical bonding, determining an element's reactivity and the types of compounds it forms. The number of valence electrons largely dictates the element's oxidation states and its ability to gain, lose, or share electrons to achieve a stable electron configuration, often resembling that of a noble gas (octet rule).
Chromium's Electronic Configuration: An Unexpected Twist
Chromium's atomic number is 24, meaning it has 24 electrons. Based on the Aufbau principle and Hund's rule, one might expect its electronic configuration to be 1s²2s²2p⁶3s²3p⁶4s²3d⁴. However, this isn't the case. The actual electronic configuration of a chromium atom is 1s²2s²2p⁶3s²3p⁶3d⁵4s¹.
Why the Anomaly? The Stability of a Half-Filled d Subshell
The unexpected configuration arises from the exceptional stability associated with a half-filled d subshell. A half-filled d subshell (d⁵) and a fully filled d subshell (d¹⁰) possess extra stability due to factors such as:
- Exchange Energy: Electrons with parallel spins in degenerate orbitals (orbitals of the same energy) experience a repulsive force, but this is more than compensated for by exchange energy, which is a quantum mechanical effect that stabilizes the system. A half-filled d subshell maximizes this exchange energy.
- Symmetrical Electron Distribution: A half-filled d subshell leads to a more symmetrical distribution of electron density, contributing to increased stability.
This increased stability outweighs the slight energy advantage of having a completely filled 4s subshell (4s²). Therefore, chromium "promotes" one electron from the 4s orbital to the 3d orbital, resulting in the observed 3d⁵4s¹ configuration.
Determining the Number of Valence Electrons in Chromium
Now, the crucial question: how many valence electrons does chromium have? This is a bit more nuanced than it first appears. While the 4s electrons are traditionally considered valence electrons, the 3d electrons in transition metals like chromium also participate in bonding, particularly in higher oxidation states.
Therefore, we can consider chromium to have six valence electrons, encompassing both the single 4s electron and the five 3d electrons. However, it's important to understand that the extent to which the 3d electrons participate in bonding can vary depending on the chemical environment and the oxidation state of the chromium ion.
Oxidation States and Valence Electrons: A Deeper Look
Chromium exhibits several oxidation states, the most common being +2, +3, and +6. The number of valence electrons involved in bonding differs for each:
- Cr²⁺ (Chromium(II) ion): Loses two electrons (the 4s¹ and one 3d electron), effectively having four remaining d electrons, though these might not all actively participate in bonding in every compound.
- Cr³⁺ (Chromium(III) ion): Loses three electrons (the 4s¹ and two 3d electrons), resulting in three d electrons. This is a very common and stable oxidation state for chromium.
- Cr⁶⁺ (Chromium(VI) ion): Loses all six valence electrons (the 4s¹ and five 3d electrons). This oxidation state is highly oxidizing and usually exists as chromate (CrO₄²⁻) or dichromate (Cr₂O₇²⁻) ions.
It's essential to remember that the concept of valence electrons in transition metals is more complex than in main group elements. The involvement of d electrons in bonding varies, leading to a range of oxidation states and diverse chemical behavior.
Chemical Implications of Chromium's Electronic Configuration
The unique electronic configuration of chromium profoundly impacts its chemical properties and reactivity. Several key features highlight this connection:
- Variable Oxidation States: The ability of chromium to readily lose different numbers of electrons explains its diverse oxidation states (+2, +3, +4, +5, +6). This versatility makes chromium an essential component in various chemical reactions and catalytic processes.
- Complex Formation: Chromium readily forms complexes with ligands (molecules or ions that bond to the central metal ion). The partially filled d orbitals enable the formation of numerous coordination complexes with varying geometries and colors, a key factor in chromium's use in pigments and dyes.
- Catalytic Activity: Chromium's ability to exist in multiple oxidation states makes it an effective catalyst in several industrial processes. For instance, chromium compounds are used in polymerization reactions and in the production of various organic chemicals.
- Corrosion Resistance: The addition of chromium to iron forms stainless steel, exhibiting excellent corrosion resistance. This is linked to the formation of a passive oxide layer (Cr₂O₃) on the steel surface, which protects it from further oxidation.
Chromium in Everyday Life: A Diverse Applications Overview
The unique properties arising from its electronic configuration translate into a wide range of applications for chromium and its compounds:
- Stainless Steel: As mentioned earlier, chromium is a critical component of stainless steel, providing superior corrosion resistance and making it suitable for various applications, from cutlery and kitchen appliances to medical instruments and automotive parts.
- Pigments and Dyes: Chromium compounds are widely used as pigments in paints, inks, and plastics, providing vibrant colors ranging from yellows and oranges to greens.
- Tanning Leather: Chromium salts are used in the tanning of leather, altering the protein structure and making it more durable and resistant to decay.
- Metallurgy: Chromium is also used in various alloys to enhance their strength, hardness, and corrosion resistance.
- Catalysis: Chromium-based catalysts play important roles in several chemical processes, notably in the production of plastics and other organic compounds.
Conclusion: The Significance of Electronic Configuration
Understanding the electronic configuration of an element is pivotal in comprehending its chemical behavior. Chromium's unusual electronic configuration, driven by the stability of a half-filled d subshell, significantly influences its properties. This leads to its variable oxidation states, ability to form complexes, catalytic activity, and ultimately, its widespread applications in various industries and everyday life. While the precise number of valence electrons involved in bonding can vary depending on the specific chemical environment, appreciating the overall contribution of both 3d and 4s electrons provides a comprehensive understanding of chromium's unique chemistry and its diverse applications. This knowledge is fundamental to advancements in materials science, catalysis, and other fields relying on chromium's remarkable properties.
Latest Posts
Latest Posts
-
A Common Flashlight Bulb Is Rated At
Mar 19, 2025
-
Log X Log X 3 1
Mar 19, 2025
-
What Is The Molecular Geometry For Bf3
Mar 19, 2025
-
The Subatomic Particle With A Positive Charge Is The
Mar 19, 2025
-
Select All Of The Statements Which Are True About Rainforests
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Cr Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
