The Subatomic Particle With A Positive Charge Is The
News Leon
Mar 19, 2025 · 7 min read
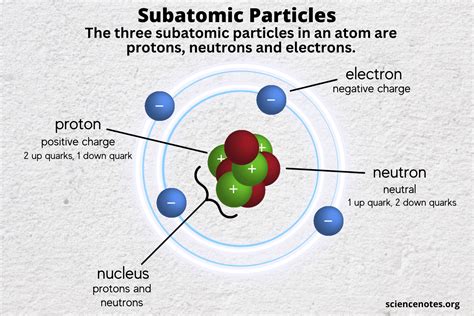
Table of Contents
The Subatomic Particle with a Positive Charge Is the Proton: A Deep Dive into its Properties and Significance
The subatomic particle with a positive charge is the proton. While seemingly simple, the proton is a fundamental building block of matter and plays a crucial role in the structure and behavior of atoms, nuclei, and the universe itself. Understanding the proton is key to comprehending chemistry, physics, and even cosmology. This article will delve deep into the properties, characteristics, and significance of the proton, exploring its historical discovery, its internal structure, its role in nuclear reactions, and its importance in various scientific fields.
The Discovery and Early Understanding of the Proton
The discovery of the proton wasn't a single "eureka" moment but rather a gradual unveiling through various experiments and theoretical developments. While the concept of the atom had been debated for centuries, the understanding of its constituents remained elusive until the late 19th and early 20th centuries. Experiments involving the passage of electric current through gases, like those conducted by Eugen Goldstein in 1886, revealed the existence of positively charged particles, which he termed "canal rays." These rays, later understood to be streams of protons, were observed traveling in the opposite direction to the cathode rays (electrons).
Ernest Rutherford's groundbreaking gold foil experiment in 1911 provided further evidence. By bombarding a thin gold foil with alpha particles (helium nuclei), Rutherford observed that some particles were deflected at large angles, suggesting the existence of a small, dense, positively charged nucleus at the center of the atom. This nucleus, he hypothesized, contained most of the atom's mass. While Rutherford didn't explicitly identify the positive particle as the proton, his work laid the foundation for its later characterization.
In 1919, Rutherford himself performed experiments where he bombarded nitrogen gas with alpha particles, resulting in the emission of protons. This was the first direct observation and identification of protons as distinct particles. He demonstrated that the alpha particles knocked protons out of the nitrogen nuclei, confirming their presence as fundamental constituents of atomic nuclei. This discovery solidified the proton's place in the atomic model and revolutionized our understanding of matter.
The Properties of the Proton
The proton possesses several key properties that define its behavior and interactions:
1. Charge:
The proton carries a single unit of positive electric charge (+1e), exactly opposite and equal in magnitude to the charge of an electron (-1e). This positive charge is crucial for the electrostatic attraction between protons and electrons, which holds atoms together.
2. Mass:
A proton is significantly more massive than an electron. Its mass is approximately 1,836 times that of an electron, a crucial factor in determining the behavior of atoms and nuclei. This mass is typically expressed in atomic mass units (amu), with one proton having a mass of approximately 1 amu.
3. Spin:
The proton possesses an intrinsic angular momentum called spin, which is a quantum mechanical property. Its spin is ½, classifying it as a fermion, meaning it obeys the Pauli Exclusion Principle, which dictates that no two protons can occupy the same quantum state simultaneously. This property is critical in determining the arrangement of protons within an atomic nucleus.
4. Composition:
Protons are not fundamental particles in the same way as electrons. They are composed of three quarks: two up quarks and one down quark, bound together by the strong nuclear force mediated by gluons. This internal structure gives rise to the proton's properties and explains why it has a positive charge (up quarks carry +2/3e charge, down quarks -1/3e charge). The complex dynamics within the proton are still an area of active research in particle physics.
5. Stability:
Free protons are stable particles; they do not decay into other particles spontaneously. However, protons within an atomic nucleus can participate in nuclear reactions, such as radioactive decay or nuclear fusion, leading to changes in the nucleus's composition.
The Proton's Role in Atomic Structure and Nuclear Physics
The proton's positive charge and mass are fundamental to atomic structure. The number of protons in an atom's nucleus defines its atomic number, and this number uniquely identifies the chemical element. For example, hydrogen has one proton, helium has two, and carbon has six. The protons in the nucleus attract electrons, which are negatively charged, and this electrostatic force binds the electrons to the nucleus, forming the atom. The number of neutrons in the nucleus also plays a role, but it’s the number of protons which determines the fundamental chemical properties of the element.
In nuclear physics, protons are integral to various processes. Nuclear fusion, the process that powers stars, involves the combining of protons (and neutrons) to form heavier atomic nuclei. Nuclear fission, the splitting of heavy nuclei, also involves the rearrangement of protons and neutrons. These processes are accompanied by enormous energy releases, making them significant in energy production and weaponry.
The strong nuclear force, one of the four fundamental forces of nature, is responsible for holding the protons (and neutrons) together within the atomic nucleus, overcoming the electrostatic repulsion between the positively charged protons. This force is much stronger than the electromagnetic force at short distances but falls off rapidly with distance. Understanding the strong force is crucial to understanding the stability and properties of atomic nuclei.
The Proton and Other Subatomic Particles
The proton is just one of many subatomic particles that make up the universe. It is often contrasted with other particles to highlight its unique properties. For instance:
- Electrons: Unlike protons, electrons are fundamental particles (not composed of smaller constituents) and carry a single negative charge. They have significantly less mass than protons and occupy the space surrounding the atomic nucleus.
- Neutrons: Neutrons are found in the atomic nucleus alongside protons. They are slightly more massive than protons and carry no net electric charge (neutral). Their presence is crucial for nuclear stability, helping to counteract the electrostatic repulsion between protons.
- Quarks: Protons are composed of quarks, which are fundamental particles that experience the strong nuclear force. There are six types of quarks: up, down, charm, strange, top, and bottom.
- Gluons: Gluons are the force-carrying particles that mediate the strong nuclear force between quarks, holding them together within protons and other hadrons.
Understanding the relationships and interactions between these various subatomic particles is crucial to comprehending the structure and behavior of matter at the most fundamental level.
The Proton in Scientific Research and Applications
The proton's properties and interactions are crucial in numerous scientific fields:
- Particle Physics: Protons serve as projectiles in high-energy particle accelerators, such as the Large Hadron Collider (LHC), used to explore the fundamental forces and particles of nature. By colliding protons at extremely high energies, physicists can probe the structure of matter and search for new particles.
- Nuclear Medicine: Proton therapy, a type of radiation therapy, uses beams of protons to target and destroy cancerous cells. The highly focused nature of proton beams minimizes damage to surrounding healthy tissues, offering a potentially more effective and less invasive treatment option compared to traditional radiation therapy.
- Nuclear Energy: Understanding proton interactions is essential in nuclear power generation and the development of controlled fusion reactors. Nuclear fission reactions, used in current nuclear power plants, involve the splitting of heavy nuclei, often releasing protons. Fusion reactions, the potential future source of clean energy, involve the combining of protons and neutrons.
- Material Science: The behavior of protons plays a crucial role in the properties of various materials. Their interactions influence the electrical conductivity, magnetic properties, and other characteristics of solids, liquids, and gases.
The Ongoing Mystery of the Proton's Structure and Behavior
Despite the significant progress in understanding protons, many mysteries remain. The precise distribution of charge and spin within the proton is still being investigated. Researchers are constantly refining models that attempt to describe the complex interactions between the quarks and gluons that constitute the proton. The ongoing research on the proton's internal structure and behavior involves sophisticated theoretical models, complex computational simulations, and high-energy experiments. These efforts aim to uncover the fundamental laws governing the behavior of matter at its most basic level. Understanding the proton is not merely an academic pursuit; it has far-reaching implications for many technological advancements and our understanding of the universe itself. From creating cleaner energy sources to developing more effective cancer treatments, the proton continues to hold immense potential for scientific and technological breakthroughs. The journey of discovering and understanding this tiny, positively charged particle is an ongoing adventure, pushing the boundaries of human knowledge and technological capabilities.
Latest Posts
Latest Posts
-
Does The I Band Shorten During Contraction
Mar 19, 2025
-
Ability To Respond To A Stimulus
Mar 19, 2025
-
What Element Has 7 Protons 8 Neutrons And 10 Electrons
Mar 19, 2025
-
A Body Is A Particular Amount Of Matter
Mar 19, 2025
-
3 Protons 4 Neutrons 3 Electrons
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about The Subatomic Particle With A Positive Charge Is The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
