What Is The Molecular Geometry For Bf3
News Leon
Mar 19, 2025 · 6 min read
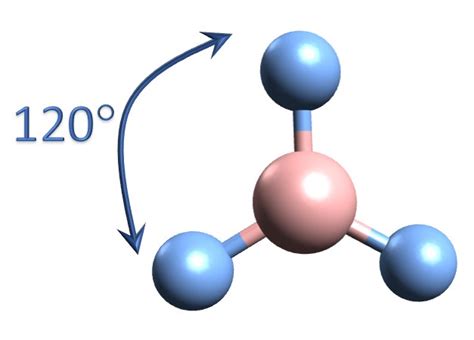
Table of Contents
What is the Molecular Geometry for BF3? A Deep Dive into Boron Trifluoride
Boron trifluoride (BF₃), a colorless, toxic gas, holds a significant place in chemistry, particularly in understanding molecular geometry and bonding. Its structure isn't just a simple arrangement of atoms; it offers a crucial example of how molecular geometry influences reactivity and properties. This article provides a comprehensive exploration of BF₃'s molecular geometry, delving into its bonding, hybridization, and implications for its chemical behavior.
Understanding Molecular Geometry
Before diving into the specifics of BF₃, let's establish a foundational understanding of molecular geometry. It refers to the three-dimensional arrangement of atoms in a molecule. This arrangement is crucial because it dictates a molecule's properties, including its polarity, reactivity, and physical state. Molecular geometry is determined by several factors, primarily the number of electron domains surrounding the central atom. These domains can be bonding pairs (shared electrons in covalent bonds) or lone pairs (unshared electrons). Models like Valence Shell Electron Pair Repulsion (VSEPR) theory are instrumental in predicting molecular geometry.
The VSEPR Theory: A Cornerstone of Molecular Geometry Prediction
The VSEPR theory postulates that electron domains around a central atom repel each other and arrange themselves to minimize this repulsion. This arrangement dictates the molecule's overall geometry. The theory considers both bonding and non-bonding electron pairs. Different arrangements of electron domains lead to distinct molecular geometries. For instance, two electron domains result in a linear geometry, three domains in a trigonal planar geometry, and four domains in a tetrahedral geometry. Understanding these basic shapes is fundamental to predicting the molecular geometry of more complex molecules.
Delving into the Structure of BF₃: A Trigonal Planar Geometry
Now, let's focus on boron trifluoride (BF₃). Boron, being in Group 13 of the periodic table, has three valence electrons. Each fluorine atom, from Group 17, contributes one electron to form a single covalent bond with boron. This results in a total of three bonding pairs surrounding the central boron atom. Crucially, there are no lone pairs on the boron atom.
The absence of lone pairs is crucial. According to VSEPR theory, three bonding pairs arranged around a central atom will minimize repulsion by adopting a trigonal planar geometry. This means that the three fluorine atoms are arranged symmetrically around the boron atom, forming a flat triangular shape with bond angles of 120°. This planar structure is a defining characteristic of BF₃ and is responsible for many of its unique properties.
Hybridization in BF₃: sp² Hybridization
The bonding in BF₃ can also be explained through the concept of hybridization. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. In BF₃, the boron atom undergoes sp² hybridization. This involves the mixing of one s orbital and two p orbitals to form three sp² hybrid orbitals. These hybrid orbitals are arranged in a trigonal planar geometry, each overlapping with a p orbital from a fluorine atom to form a sigma (σ) bond.
The remaining unhybridized p orbital on boron remains unoccupied, contributing to BF₃'s unique reactivity, as we will discuss later. The sp² hybridization provides a powerful explanation for the observed trigonal planar geometry and the strong, relatively short B-F bonds.
The Impact of Molecular Geometry on BF₃'s Properties
The trigonal planar geometry of BF₃ has profound implications for its chemical and physical properties. Let's explore some of these crucial effects:
1. Nonpolarity: A Consequence of Symmetry
The symmetrical arrangement of fluorine atoms around the boron atom in BF₃ makes the molecule nonpolar. Although each B-F bond is polar (due to the difference in electronegativity between boron and fluorine), the individual bond dipoles cancel each other out due to the molecule's symmetrical trigonal planar geometry. This nonpolarity influences its solubility and interactions with other molecules.
2. Lewis Acidity: The Role of the Empty p Orbital
One of BF₃'s most important properties is its Lewis acidity. This stems directly from the presence of the empty, unhybridized p orbital on the boron atom. This empty orbital can readily accept a lone pair of electrons from a Lewis base (a molecule with a lone pair of electrons). This electron pair acceptance forms a coordinate covalent bond, where both electrons in the bond come from the Lewis base. This Lewis acidity makes BF₃ a versatile reagent in many organic and inorganic reactions. Common examples include its use as a catalyst in Friedel-Crafts reactions.
3. Reactivity: A Direct Result of Geometry and Electronic Structure
The reactivity of BF₃ is intimately linked to its molecular geometry and electronic structure. Its ability to act as a Lewis acid drives its participation in a wide range of chemical reactions. The planar structure allows for easy approach of Lewis bases to the boron atom, facilitating the formation of adducts. The strength of the B-F bonds also influences reactivity, as the energy required to break these bonds plays a role in the overall reaction energetics.
BF₃ vs. Other Trigonal Planar Molecules
While BF₃ serves as a prime example of a trigonal planar molecule, it's important to compare it with other molecules exhibiting this geometry. Consider molecules like SO₃ (sulfur trioxide) and NO₃⁻ (nitrate ion). All three share the trigonal planar structure but differ in their bonding and properties. The differences arise primarily from the presence or absence of lone pairs on the central atom and the differing electronegativities of the constituent atoms.
Comparing BF₃, SO₃, and NO₃⁻: Similarities and Differences
While all three have a trigonal planar geometry, SO₃ and NO₃⁻ have differences compared to BF₃:
-
Lone pairs: Both SO₃ and NO₃⁻ have resonance structures involving lone pairs on the central atom (sulfur and nitrogen, respectively). This presence of lone pairs affects their Lewis acidity and overall reactivity compared to BF₃, which has no lone pairs on boron. SO₃ is still somewhat Lewis acidic, but less than BF₃. NO₃⁻ is not a Lewis acid.
-
Electronegativity: The electronegativity differences between the central atom and surrounding atoms also influence the overall polarity and reactivity. The higher electronegativity of oxygen in SO₃ and NO₃⁻ compared to fluorine in BF₃ affects the charge distribution within the molecules.
-
Bonding: The bonding in SO₃ and NO₃⁻ involves pi (π) bonding in addition to sigma (σ) bonds, a feature absent in BF₃, leading to resonance. This resonance affects bond length and strength.
These comparisons highlight that while the trigonal planar geometry is a common feature, the specific chemical behavior of a molecule is determined by a combination of its geometry, bonding, and the electronegativity of its constituent atoms.
Conclusion: A Comprehensive Understanding of BF₃
Boron trifluoride's trigonal planar molecular geometry isn't just a structural feature; it's the key to understanding its unique reactivity and chemical properties. The absence of lone pairs on boron, its sp² hybridization, and the resulting empty p orbital all contribute to its Lewis acidity, a defining characteristic that makes it a crucial reagent in various chemical processes. By comparing it with other trigonal planar molecules, we gain a deeper appreciation for the nuanced relationship between molecular geometry, bonding, and chemical behavior. The study of BF₃ provides an excellent illustration of how seemingly simple molecular structures can lead to complex and fascinating chemical reactivity. Further exploration of this molecule and related compounds will continue to reveal new insights into the intricate world of chemical bonding and molecular interactions.
Latest Posts
Latest Posts
-
Which Of The Following Antibodies Is A Pentamer
Mar 19, 2025
-
What Is The River Behind The Taj Mahal
Mar 19, 2025
-
Why Is The Heart Called A Double Pump
Mar 19, 2025
-
What Percentage Of 8 Is 64
Mar 19, 2025
-
How Many Pints In One Pound
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Geometry For Bf3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
