How Many Valence Electrons Does Chromium Have
News Leon
Mar 18, 2025 · 5 min read
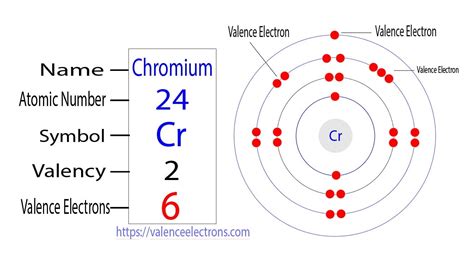
Table of Contents
How Many Valence Electrons Does Chromium Have? A Deep Dive into Electronic Configuration and Chemical Behavior
Chromium, a lustrous, hard, and brittle transition metal, finds widespread applications in various industries, from stainless steel production to the creation of pigments and catalysts. Understanding its chemical behavior is crucial to harnessing its diverse functionalities. Central to this understanding is its electronic configuration, particularly the number of valence electrons. This article will delve into the intricacies of chromium's electronic structure, explaining why determining its valence electron count isn't as straightforward as it might initially seem.
The Expected vs. The Unexpected: Chromium's Electronic Configuration
You might expect chromium (Cr), with an atomic number of 24, to have an electronic configuration of 1s²2s²2p⁶3s²3p⁶4s²3d⁴. This follows the Aufbau principle, which dictates that electrons fill orbitals in order of increasing energy. However, this isn't the actual electronic configuration observed for chromium.
The Half-Filled and Fully-Filled Stability Rule
The actual electronic configuration of chromium is 1s²2s²2p⁶3s²3p⁶4s¹3d⁵. This seemingly aberrant configuration is due to a phenomenon known as half-filled and fully-filled subshell stability. A half-filled or fully-filled d subshell is remarkably stable due to several factors:
-
Electron Exchange Energy: Electrons in a half-filled or fully-filled subshell can exchange positions, leading to a reduction in electron-electron repulsion and an increase in stability. This exchange energy is maximized when the subshell is half-filled or fully-filled.
-
Symmetrical Electron Distribution: This symmetrical distribution of electrons results in a more stable, lower-energy configuration.
-
Increased Orbital Overlap: The electrons in half-filled or fully-filled subshells experience improved orbital overlap with other atoms, leading to stronger bonding interactions.
By promoting one electron from the 4s orbital to the 3d orbital, chromium achieves a half-filled 3d subshell and a half-filled 4s subshell. This seemingly minor adjustment results in a significantly more stable electronic configuration than the configuration predicted by the Aufbau principle. This exceptional stability influences many of chromium's chemical properties.
Determining the Number of Valence Electrons
So, how many valence electrons does chromium actually possess? The answer isn't immediately clear due to the complexity of its electronic configuration. The definition of a valence electron itself requires clarification within the context of transition metals.
Valence Electrons in Transition Metals: A Nuanced Definition
Unlike main group elements, where valence electrons are primarily located in the outermost s and p orbitals, transition metals can involve d electrons in bonding. The outermost s electrons (4s in chromium's case) are always considered valence electrons. However, the involvement of d electrons in bonding varies depending on the oxidation state of the metal.
Chromium's Variable Oxidation States
Chromium exhibits several oxidation states, most commonly +2, +3, and +6. The number of valence electrons effectively involved in bonding changes depending on the oxidation state:
-
Cr(II) (Chromium(II)): In this oxidation state, chromium loses two electrons, likely from the 4s orbital. Thus, it effectively has 4 valence electrons involved in bonding (3d⁵ + 4s¹ → 3d⁴ after losing two).
-
Cr(III) (Chromium(III)): In this oxidation state, chromium loses three electrons, one from 4s and two from 3d. This leaves effectively 3 valence electrons involved in bonding (3d⁵ + 4s¹ → 3d³ after losing three).
-
Cr(VI) (Chromium(VI)): This is the highest common oxidation state. Here, chromium loses all six electrons in the 3d and 4s orbitals, resulting in zero valence electrons remaining on the Chromium ion. However, the bonding here is more accurately described using covalent interactions than simple electron loss.
Therefore, there's no single answer to the question "How many valence electrons does chromium have?". It depends heavily on the specific chemical context and its oxidation state.
The Implications of Variable Valence Electrons
Chromium's variable valence electron count is reflected in its diverse chemical behavior:
-
Formation of various colored compounds: The d electrons involved in bonding can absorb visible light, leading to the formation of intensely colored compounds. This property underlies chromium's use in pigments.
-
Catalytic activity: The ability of chromium to exist in multiple oxidation states allows it to act as a catalyst in various chemical reactions. It can readily gain and lose electrons, facilitating redox processes.
-
Formation of complex ions: Chromium readily forms complex ions with ligands, where the d orbitals participate actively in coordination bonds.
-
Alloying properties: The diverse oxidation states and bonding capabilities of chromium contribute to its usefulness in alloying, particularly in stainless steel, where it enhances corrosion resistance.
Beyond Valence Electrons: Exploring Chromium's Chemical Properties
While the number of valence electrons is a key factor in determining chemical behavior, it's not the only determinant. Other factors influence chromium's reactivity:
-
Atomic Radius: Chromium's relatively small atomic radius influences its ability to form strong bonds.
-
Electronegativity: Chromium's intermediate electronegativity contributes to its ability to form both ionic and covalent bonds.
-
Ionization Energies: The energy required to remove electrons from chromium influences its oxidation states.
Conclusion: A Versatile Metal with a Complex Electronic Structure
Chromium's electronic configuration is more complex than initially anticipated due to the enhanced stability of half-filled and fully-filled subshells. This complexity leads to a range of oxidation states and a correspondingly variable number of valence electrons involved in bonding. It's not just the number of valence electrons but the interplay of its electronic structure with other atomic properties that leads to chromium's diverse and crucial roles in various chemical and industrial processes. Understanding the nuances of its electronic structure is key to comprehending its versatile chemical behavior and its myriad applications. While a definitive answer to "How many valence electrons does chromium have?" is context-dependent, this comprehensive exploration illustrates the importance of considering both the standard Aufbau principle and the subtle yet significant exceptions that shape the remarkable properties of this transition metal. The seemingly simple question uncovers a rich and fascinating tapestry of chemical behavior.
Latest Posts
Latest Posts
-
Find The Surface Area Of The Square Pyramid Shown Below
Mar 19, 2025
-
A Cell In A Hypertonic Solution Will
Mar 19, 2025
-
What Is 25 Percent Of 25
Mar 19, 2025
-
How Do You Make A Magnet At Home
Mar 19, 2025
-
A Decrease In Demand And An Increase In Supply Will
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Chromium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
