How Many Pi And Sigma Bonds In A Triple Bond
News Leon
Mar 27, 2025 · 6 min read
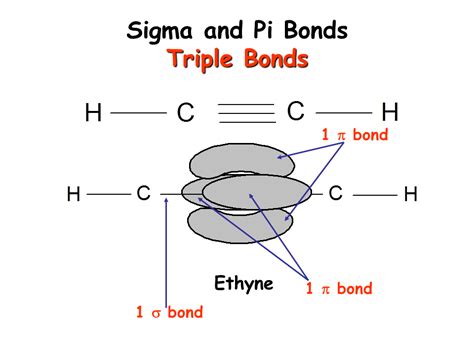
Table of Contents
How Many Pi and Sigma Bonds in a Triple Bond? A Deep Dive into Chemical Bonding
Understanding chemical bonding is fundamental to grasping the behavior of molecules. Among the various types of bonds, triple bonds stand out for their strength and unique electronic structure. This article will delve deep into the composition of a triple bond, specifically addressing the question: How many pi and sigma bonds are in a triple bond? We'll explore the fundamental principles behind sigma and pi bonds, examine the molecular orbital theory that underpins their formation, and look at examples to solidify our understanding.
Understanding Sigma (σ) and Pi (π) Bonds
Before we dissect a triple bond, let's establish a clear understanding of sigma and pi bonds. These are the two primary types of covalent bonds, arising from the overlap of atomic orbitals.
Sigma (σ) Bonds: The Foundation of Covalent Bonds
A sigma bond is formed by the head-on overlap of atomic orbitals. This means the orbitals directly overlap along the internuclear axis – the imaginary line connecting the two bonded atoms. Sigma bonds are stronger than pi bonds because of this direct, maximum overlap. They are also rotationally symmetric around the internuclear axis. This means the bond can freely rotate without significantly impacting its strength or stability. A single bond between two atoms always consists of one sigma bond.
Pi (π) Bonds: Adding Strength and Stability
Pi bonds, on the other hand, are formed by the sideways overlap of atomic orbitals. This overlap occurs above and below (or in front and behind, depending on the orientation) the internuclear axis. This sideways overlap results in a weaker bond compared to a sigma bond due to less effective orbital overlap. Critically, pi bonds are characterized by a node – a region of zero electron density – along the internuclear axis. Because of this nodal plane, pi bonds restrict rotation around the internuclear axis. This restricted rotation contributes to the rigidity of molecules containing pi bonds.
The Structure of a Triple Bond: One Sigma, Two Pi
Now, let's address the central question: How many pi and sigma bonds are in a triple bond?
A triple bond consists of one sigma bond and two pi bonds. This combination represents the strongest type of covalent bond, resulting in a short bond length and high bond energy.
Let's visualize this using the classic example of nitrogen gas (N₂):
- Each nitrogen atom contributes five valence electrons.
- One sigma bond is formed by the head-on overlap of one sp hybridized orbital from each nitrogen atom. This forms the strong, foundational bond along the internuclear axis.
- Two pi bonds are formed by the sideways overlap of two sets of p orbitals from each nitrogen atom. These two pi bonds exist above and below (or in front and behind) the sigma bond, reinforcing the bond's strength but restricting rotation.
Molecular Orbital Theory: A Deeper Look
Molecular orbital theory (MOT) offers a more sophisticated explanation for the formation of triple bonds. Instead of considering individual atomic orbitals, MOT considers the combination of atomic orbitals to form molecular orbitals that encompass the entire molecule.
In the case of a triple bond, like that in N₂, we see the formation of:
- One bonding sigma molecular orbital: formed by the constructive interference of two sp hybrid orbitals. This orbital is lower in energy than the constituent atomic orbitals.
- Two bonding pi molecular orbitals: formed by the constructive interference of two sets of p orbitals. These orbitals are also lower in energy than the constituent atomic orbitals.
- One antibonding sigma molecular orbital: formed by the destructive interference of two sp hybrid orbitals. This orbital is higher in energy than the constituent atomic orbitals.
- Two antibonding pi molecular orbitals: formed by the destructive interference of two sets of p orbitals. These orbitals are also higher in energy than the constituent atomic orbitals.
The electrons fill the lower-energy bonding orbitals first, resulting in the strong, stable triple bond. The antibonding orbitals remain unoccupied (in the ground state).
Examples of Triple Bonds in Organic and Inorganic Chemistry
Triple bonds are prevalent in many molecules, playing crucial roles in their reactivity and properties.
Alkynes: The Carbon-Carbon Triple Bond
Alkynes are hydrocarbons characterized by the presence of at least one carbon-carbon triple bond. The simplest alkyne is ethyne (acetylene), with the formula C₂H₂. The triple bond in ethyne consists of one sigma bond and two pi bonds, just like in the nitrogen molecule. The presence of the triple bond significantly impacts the alkyne's reactivity, making it susceptible to addition reactions.
Cyanides and Nitriles: Carbon-Nitrogen Triple Bonds
Cyanides and nitriles contain a carbon-nitrogen triple bond (C≡N). This triple bond is remarkably strong and polar, contributing to the unique properties of these compounds. The nitrogen atom is more electronegative, resulting in a partial positive charge on the carbon and a partial negative charge on the nitrogen. This polarity makes these compounds reactive and good ligands in coordination chemistry.
Other Inorganic Examples
Triple bonds are not limited to organic chemistry. Many inorganic molecules also feature triple bonds. Examples include:
- Carbon monoxide (CO): This molecule has a strong carbon-oxygen triple bond, contributing to its stability and toxicity.
- Dinitrogen (N₂): As discussed earlier, this is a classic example with a robust nitrogen-nitrogen triple bond.
Importance of Triple Bonds in Various Fields
The strong and unique nature of triple bonds has significant implications in various scientific and technological fields:
- Materials Science: The strength and rigidity of triple bonds make them crucial components of advanced materials, including polymers and high-performance composites.
- Organic Synthesis: Understanding the reactivity of triple bonds is vital for designing and executing organic synthesis reactions. Triple bonds can be strategically introduced or modified to create complex molecules with desired properties.
- Medicinal Chemistry: Molecules containing triple bonds often exhibit unique biological activities, leading to their use in drug discovery and development.
- Industrial Processes: Triple bonds are involved in various industrial processes, such as the production of plastics and synthetic fuels.
Conclusion: A Firm Grasp on Triple Bond Structure
In conclusion, a triple bond is a powerful and versatile form of covalent bonding, comprised of one sigma bond and two pi bonds. This combination of bonding types accounts for the short bond lengths, high bond energies, and unique properties of molecules featuring triple bonds. Understanding the structure and behavior of triple bonds is paramount in several scientific disciplines and technological applications. Whether you're studying organic chemistry, inorganic chemistry, or materials science, a thorough grasp of sigma and pi bonds, and their contribution to triple bond formation, is essential for a deeper understanding of molecular structure and reactivity.
Latest Posts
Latest Posts
-
The Pectoral Girdle Consists Of The
Mar 30, 2025
-
Variable Cost Per Unit Of Output Produced Is
Mar 30, 2025
-
Why Does Anaerobic Respiration Yield Less Energy Than Aerobic Respiration
Mar 30, 2025
-
The Functional Unit Of Skeletal Muscle Is
Mar 30, 2025
-
In Meiosis Homologous Chromosomes Are Separated During
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Pi And Sigma Bonds In A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
