How Many Elements Occur Naturally On The Earth
News Leon
Mar 31, 2025 · 5 min read
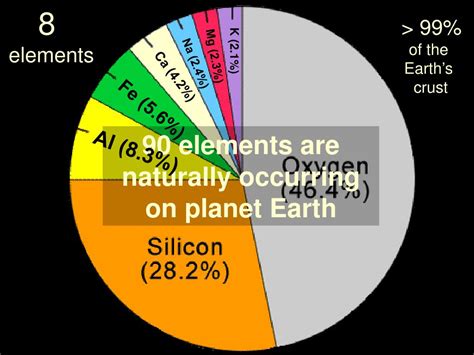
Table of Contents
How Many Elements Occur Naturally on Earth? Unraveling the Periodic Table's Terrestrial Mystery
The Earth, our vibrant and diverse planet, is a treasure trove of chemical elements. But how many of these fundamental building blocks of matter actually occur naturally within our planet's crust, oceans, and atmosphere? The answer isn't as simple as a single number, and delving into the specifics reveals a fascinating story about planetary formation, chemical processes, and the very nature of existence.
The Periodic Table: A Roadmap to the Elements
The periodic table, that iconic chart organizing all known elements, serves as our primary guide. Each element, represented by its unique symbol and atomic number (the number of protons in its nucleus), possesses distinct physical and chemical properties. While over 118 elements are currently recognized, the number found naturally on Earth is significantly smaller. Understanding this distinction is crucial to answering our central question.
Naturally Occurring vs. Synthetic Elements
The key difference lies in the origin of the element. Naturally occurring elements are those found in nature, formed through various geological processes or existing since the Earth's formation. Synthetic elements, conversely, are created artificially in laboratories through nuclear reactions. These synthetic elements are highly unstable and often decay rapidly.
The Count: A Nuance of Definition
Determining the exact number of naturally occurring elements requires careful consideration. The number often cited is around 90-94 elements. This slight variation stems from several factors:
- Trace amounts: Some elements exist in such minuscule quantities that their natural occurrence is debatable. Their presence might be more of a geological anomaly than a substantial contribution to Earth's composition. This ambiguity affects the precise count.
- Radioactive decay: Certain elements are radioactive, undergoing decay into other elements over time. The original parent element might be considered naturally occurring, even though its stable daughter product becomes more prevalent over geological ages.
- Anthropogenic influence: Human activity, particularly nuclear testing and industrial processes, has introduced some elements into the environment in quantities that weren't naturally present. The impact of this contamination on the "natural" classification is another point of discussion.
The Major Players: Abundant Elements Shaping Our World
Despite the complexities, some elements dominate Earth's composition. These abundant elements form the bedrock of our planet and are integral to various geological formations and biological processes:
Oxygen (O): The Abundant Champion
Oxygen reigns supreme, making up approximately 46% of the Earth's crust by mass. It’s a vital component of rocks, minerals, water, and the atmosphere, playing a crucial role in respiration and countless chemical reactions.
Silicon (Si): The Rock-Forming Giant
Silicon, at around 28%, is the second most abundant element. It’s a fundamental building block of silicates, the dominant minerals in the Earth's crust. These silicates form a wide variety of rocks, from granite to basalt.
Aluminum (Al), Iron (Fe), Calcium (Ca), and More
Aluminum (Al), Iron (Fe), Calcium (Ca), Sodium (Na), Potassium (K), and Magnesium (Mg) also represent significant portions of the Earth's crust, contributing to its diverse mineralogy and geological features. These elements are crucial constituents in numerous rocks and minerals.
The Rarest of the Rare: Elements in Trace Amounts
While some elements are readily available, others exist in minuscule amounts, often as trace impurities within minerals. These trace elements, though present in small quantities, can have significant geological or biological implications. For instance:
- Platinum (Pt) and Gold (Au): These precious metals are highly valued for their rarity and use in various industries. They are found in low concentrations in certain ore deposits.
- Rare Earth Elements (REEs): This group of 17 elements, including lanthanum (La), cerium (Ce), and neodymium (Nd), are crucial in modern technology, yet their distribution is uneven and often requires extensive mining efforts.
- Technetium (Tc) and Promethium (Pm): These two elements are notable for their absence in Earth's crust. They are radioactive and have extremely short half-lives, preventing their accumulation in naturally occurring mineral deposits.
The Influence of Geological Processes: Shaping Elemental Distribution
The distribution of elements on Earth isn't uniform. Geological processes play a vital role in concentrating or dispersing them:
- Plate tectonics: The movement of Earth's tectonic plates contributes to the formation of mountains, volcanoes, and ocean basins, significantly influencing the distribution of elements. Volcanic activity releases elements from the Earth's mantle, while weathering and erosion redistribute elements across the surface.
- Hydrothermal vents: These deep-sea vents release hot, mineral-rich fluids, creating unique environments where certain elements are concentrated. These vents play a role in the cycling of various elements, including sulfur and metals.
- Sedimentation: The settling of sediments in oceans and lakes contributes to the formation of sedimentary rocks, leading to the concentration of specific elements in particular locations.
Beyond the Crust: Exploring the Mantle and Core
Our discussion has mainly focused on the Earth's crust, the outermost layer readily accessible to us. However, the Earth's mantle and core contain significant quantities of elements, although their exact composition is still being researched. The core, primarily composed of iron and nickel, holds a substantial portion of these elements.
The Ongoing Quest for Knowledge
Our understanding of the Earth's elemental composition is constantly evolving. Advances in analytical techniques, coupled with exploration of remote areas and deep-sea environments, continue to refine our knowledge of element distribution and abundance. New discoveries are made regularly, further enriching our understanding of this fundamental aspect of our planet.
Conclusion: A Dynamic and Complex Picture
The question of how many elements occur naturally on Earth doesn't have a simple numerical answer. The number typically quoted – around 90-94 – is an approximation reflecting the complexities of elemental distribution, the challenges of detection at trace levels, and the evolving definitions related to "naturally occurring." The abundance and distribution of these elements are intricately linked to the Earth's geological history, ongoing processes, and the forces that have shaped our planet over billions of years. This dynamic interplay continues to shape the diversity and richness of our planet's elemental landscape, presenting a fascinating and ongoing area of scientific investigation. The periodic table serves not only as a catalog of the elements but also as a roadmap for further exploration of this endlessly intriguing natural phenomenon.
Latest Posts
Latest Posts
-
What Do All Acids Have In Common
Apr 02, 2025
-
Glucose Dissolves In Water Because It
Apr 02, 2025
-
How Many Electrons Are In Mg 2
Apr 02, 2025
-
What Is The Opposite Of Acute
Apr 02, 2025
-
Binary Search Tree Worst Case Time Complexity
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Elements Occur Naturally On The Earth . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
