How Many Atoms Are In Nahco3
News Leon
Mar 22, 2025 · 4 min read
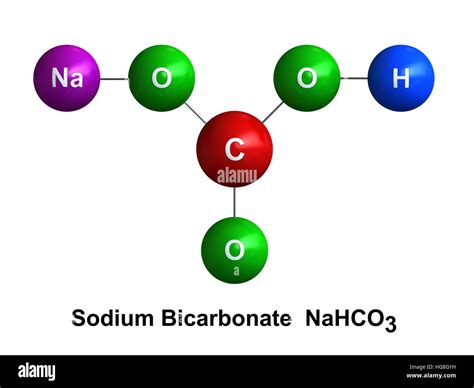
Table of Contents
How Many Atoms Are in NaHCO₃ (Sodium Bicarbonate)? A Deep Dive into Molecular Composition
Baking soda, scientifically known as sodium bicarbonate (NaHCO₃), is a ubiquitous household item with a surprisingly complex molecular structure. Understanding its composition, down to the number of atoms it contains, requires a basic grasp of chemistry and Avogadro's number. This article will delve into exactly how many atoms are in NaHCO₃, exploring the concepts behind the calculation and expanding on the broader implications of this seemingly simple question.
Understanding the Chemical Formula: NaHCO₃
Before we calculate the number of atoms, let's break down the chemical formula itself: NaHCO₃. This formula tells us the types and relative quantities of atoms present in one molecule of sodium bicarbonate:
- Na (Sodium): Represents one sodium atom.
- H (Hydrogen): Represents one hydrogen atom.
- C (Carbon): Represents one carbon atom.
- O₃ (Oxygen): Represents three oxygen atoms.
Therefore, a single molecule of NaHCO₃ contains a total of 6 atoms. This is the foundation for all further calculations.
Calculating Atoms in a Single Molecule
As established above, one molecule of NaHCO₃ contains six atoms. This is a simple but crucial step. The complexity arises when dealing with larger quantities of NaHCO₃, which is the typical scenario in real-world applications.
Scaling Up: From Molecules to Moles
To accurately determine the number of atoms in a larger quantity of NaHCO₃, we need to introduce the concept of the mole. A mole is a unit of measurement in chemistry that represents a specific number of particles, namely Avogadro's number (approximately 6.022 x 10²³). This means one mole of any substance contains 6.022 x 10²³ particles (atoms, molecules, ions, etc.).
The molar mass of NaHCO₃ is crucial for scaling up our calculations. To determine the molar mass, we add the atomic masses of each element present in the molecule:
- Na: 22.99 g/mol
- H: 1.01 g/mol
- C: 12.01 g/mol
- O₃: 3 * 16.00 g/mol = 48.00 g/mol
Adding these together, we get a molar mass of approximately 84.01 g/mol for NaHCO₃. This means that 84.01 grams of NaHCO₃ contain one mole (6.022 x 10²³ molecules) of NaHCO₃.
Calculating Atoms in a Given Mass of NaHCO₃
Let's say we want to determine the number of atoms in 1 gram of NaHCO₃. We can use the following steps:
-
Calculate the number of moles: We divide the given mass (1 gram) by the molar mass (84.01 g/mol):
1 g / 84.01 g/mol ≈ 0.0119 moles
-
Calculate the number of molecules: We multiply the number of moles by Avogadro's number:
0.0119 moles * 6.022 x 10²³ molecules/mol ≈ 7.16 x 10²¹ molecules
-
Calculate the total number of atoms: Since each molecule contains 6 atoms, we multiply the number of molecules by 6:
7.16 x 10²¹ molecules * 6 atoms/molecule ≈ 4.30 x 10²² atoms
Therefore, there are approximately 4.30 x 10²² atoms in 1 gram of NaHCO₃.
Exploring the Implications: Real-World Applications
The seemingly simple calculation of atoms in NaHCO₃ has far-reaching implications across various scientific disciplines and everyday applications. Understanding the molecular composition is fundamental to:
-
Baking and Cooking: The leavening action of baking soda relies on the release of carbon dioxide gas when it reacts with acidic ingredients. Knowing the number of molecules and atoms helps in precise recipe formulation and understanding the chemical reactions involved.
-
Medicine and Pharmaceuticals: Sodium bicarbonate is used in various medical applications, including as an antacid to neutralize stomach acid and as a component in intravenous fluids. Accurate calculation of dosage requires a thorough understanding of its molecular composition and molar mass.
-
Environmental Science: Sodium bicarbonate plays a role in various environmental processes. Its presence and concentration in natural systems can be analyzed to understand geological formations and ecological balances.
-
Industrial Applications: Sodium bicarbonate is used in numerous industrial processes, such as fire suppression, water treatment, and cleaning. Understanding its molecular structure is crucial for optimizing its use in various applications.
-
Agricultural Applications: Sodium bicarbonate can be used in agriculture to adjust soil pH and in pest control. Understanding its interactions with plants and soil requires knowledge of its atomic composition.
Beyond the Basics: Isotopes and Atomic Mass
The calculations presented above use standard atomic masses. However, it's crucial to note that elements exist as isotopes – atoms with the same number of protons but different numbers of neutrons. These isotopes have slightly different masses. While the differences are usually negligible for most practical purposes, considering isotopic abundances can lead to more precise calculations of molar mass and, consequently, the number of atoms.
Conclusion: A Simple Calculation, Wide-Ranging Impact
While calculating the number of atoms in NaHCO₃ might seem like a simple exercise in stoichiometry, it underpins a deeper understanding of chemistry and its applications in the real world. From baking a cake to understanding complex chemical reactions in industrial processes, the knowledge of atomic composition is essential. This article has provided a comprehensive guide to this calculation, emphasizing the importance of Avogadro's number, molar mass, and the broader significance of understanding molecular structure. Remember that the accuracy of these calculations depends on the precision of the values used, including atomic masses and Avogadro's number. Further exploration into isotopic variations can enhance the accuracy of these calculations for high-precision applications.
Latest Posts
Latest Posts
-
Chromium Has An Atomic Mass Of 51 9961
Mar 23, 2025
-
A Meter Stick Balances Horizontally On A Knife Edge
Mar 23, 2025
-
Find The Lettered Angle In Each Case
Mar 23, 2025
-
Which Of The Following Reactions Does Not Involve Oxidation Reduction
Mar 23, 2025
-
The Gas Which Turns Lime Water Milky Is
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Nahco3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
