Which Of The Following Reactions Does Not Involve Oxidation-reduction
News Leon
Mar 23, 2025 · 6 min read
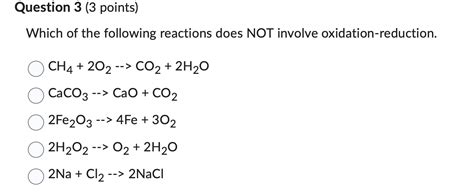
Table of Contents
Which of the Following Reactions Does Not Involve Oxidation-Reduction? A Deep Dive into Redox Reactions and Beyond
Oxidation-reduction reactions, or redox reactions, are fundamental chemical processes involving the transfer of electrons between species. Understanding redox reactions is crucial in various fields, from biology and chemistry to environmental science and materials science. But not all chemical reactions are redox reactions. This article will delve into the core principles of redox reactions, explore various reaction types, and definitively answer the question: which reactions don't involve oxidation-reduction?
Understanding Oxidation and Reduction
Before we identify non-redox reactions, let's solidify our understanding of the core concepts: oxidation and reduction. These processes are always coupled; one cannot occur without the other.
Oxidation: Loss of Electrons
Oxidation is defined as the loss of electrons by an atom, ion, or molecule. This loss of electrons results in an increase in the oxidation state (oxidation number) of the species involved. Think of it as something "giving away" electrons.
Example: The oxidation of iron (Fe) to iron(III) ion (Fe³⁺):
Fe → Fe³⁺ + 3e⁻
Here, iron loses three electrons, increasing its oxidation state from 0 to +3.
Reduction: Gain of Electrons
Reduction is the gain of electrons by an atom, ion, or molecule. This gain of electrons results in a decrease in the oxidation state of the species. This is the opposite of oxidation; something is "receiving" electrons.
Example: The reduction of copper(II) ion (Cu²⁺) to copper (Cu):
Cu²⁺ + 2e⁻ → Cu
Here, copper(II) ion gains two electrons, decreasing its oxidation state from +2 to 0.
Identifying Redox Reactions: Key Indicators
Several indicators can help you identify whether a reaction is a redox reaction:
-
Changes in Oxidation States: The most definitive way to identify a redox reaction is to track the oxidation states of all atoms involved in the reaction. If the oxidation state of at least one atom changes, it's a redox reaction.
-
Presence of Oxidizing and Reducing Agents: Redox reactions involve an oxidizing agent, which accepts electrons and gets reduced, and a reducing agent, which donates electrons and gets oxidized. Identifying these agents is a strong indicator of a redox reaction.
-
Electron Transfer: The explicit transfer of electrons is a clear sign of a redox reaction. This is often evident in the balanced chemical equation.
Types of Reactions that Are Redox Reactions
Many common chemical reactions fall under the umbrella of redox reactions. Some examples include:
1. Combustion Reactions:
Combustion reactions are rapid redox reactions that involve the reaction of a substance with an oxidant, usually oxygen, to produce heat and light. The fuel (often a hydrocarbon) is oxidized, and oxygen is reduced.
Example: The combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
In this reaction, carbon in methane is oxidized (+4 in CO₂ compared to -4 in CH₄), and oxygen is reduced (from 0 to -2).
2. Single Displacement Reactions:
Single displacement reactions involve one element replacing another element in a compound. These are often redox reactions.
Example: The reaction of zinc with hydrochloric acid:
Zn + 2HCl → ZnCl₂ + H₂
Zinc is oxidized (0 to +2), and hydrogen is reduced (+1 to 0).
3. Corrosion:
Corrosion, the gradual destruction of materials due to chemical reactions with their environment, is a classic example of a redox process. Metals, particularly iron, are oxidized in the presence of oxygen and moisture, forming metal oxides (rust).
4. Respiration and Photosynthesis (Biological Redox):
These vital biological processes are driven by redox reactions. In respiration, glucose is oxidized, and oxygen is reduced. Photosynthesis reverses this process, oxidizing water and reducing carbon dioxide.
Types of Reactions that are Not Redox Reactions
Now, let's focus on the main point of this article: identifying reactions that do not involve the transfer of electrons.
1. Acid-Base Reactions (Neutralization Reactions):
Acid-base reactions, also known as neutralization reactions, involve the transfer of protons (H⁺) between an acid and a base. No change in oxidation states occurs. The proton transfer is not an electron transfer.
Example: The reaction between hydrochloric acid and sodium hydroxide:
HCl + NaOH → NaCl + H₂O
In this reaction, the hydrogen ion from HCl is transferred to the hydroxide ion from NaOH, forming water. The oxidation states of all atoms remain unchanged.
2. Precipitation Reactions:
Precipitation reactions involve the formation of an insoluble solid (precipitate) when two aqueous solutions are mixed. These reactions usually involve the combination of ions to form an insoluble ionic compound. There is no change in oxidation states.
Example: The reaction between silver nitrate and sodium chloride:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
The silver and chloride ions combine to form the insoluble silver chloride precipitate. No electron transfer or change in oxidation states occurs.
3. Double Displacement Reactions (Metathesis Reactions):
Double displacement reactions involve the exchange of ions between two compounds. Like precipitation reactions, these typically do not involve changes in oxidation states.
Example: The reaction between potassium chloride and silver nitrate:
KCl(aq) + AgNO₃(aq) → KNO₃(aq) + AgCl(s)
Ions are exchanged, but no electron transfer occurs.
4. Isomerization Reactions:
Isomerization reactions involve the rearrangement of atoms within a molecule to form a structural isomer. These reactions do not involve any changes in oxidation states as the bonding and number of atoms are preserved.
Example: The isomerization of cis-2-butene to trans-2-butene. The atoms are simply rearranged in space, there's no electron transfer.
5. Complexation Reactions (Coordination Reactions):
Complexation reactions involve the formation of a complex ion from a central metal ion and ligands (molecules or ions that bond to the metal ion). While the coordination number and geometry of the metal ion might change, these reactions generally do not involve a change in oxidation state of the central metal ion unless the ligand itself is involved in a redox reaction.
Distinguishing Redox from Non-Redox: A Practical Approach
To confidently determine if a reaction is redox or not, follow these steps:
-
Assign Oxidation States: Carefully assign oxidation states to all atoms in both the reactants and products.
-
Compare Oxidation States: Compare the oxidation states of each atom in the reactants to their oxidation states in the products.
-
Identify Changes: If the oxidation state of at least one atom changes, the reaction is a redox reaction. If there are no changes in oxidation states, the reaction is not a redox reaction.
Conclusion
Understanding the difference between redox and non-redox reactions is fundamental to mastering chemistry. While many chemical processes involve the transfer of electrons, many others, like acid-base reactions, precipitation reactions, and isomerizations, do not. By carefully analyzing oxidation states and identifying electron transfer, you can confidently classify any given reaction. Remember, the key lies in observing changes in oxidation states – the definitive indicator of a redox reaction. Mastering this skill will significantly enhance your comprehension of chemical reactions and their diverse applications.
Latest Posts
Latest Posts
-
In Which Stage Of Meiosis Crossing Over Takes Place
Mar 24, 2025
-
Which Of The Following Is Not A Major Nutrient
Mar 24, 2025
-
The Figure Gives The One Dimensional Potential Energy
Mar 24, 2025
-
Saying The Opposite Of What You Mean
Mar 24, 2025
-
Maximum Number Of Electrons In D Orbital
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Reactions Does Not Involve Oxidation-reduction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
