Maximum Number Of Electrons In D Orbital
News Leon
Mar 24, 2025 · 6 min read
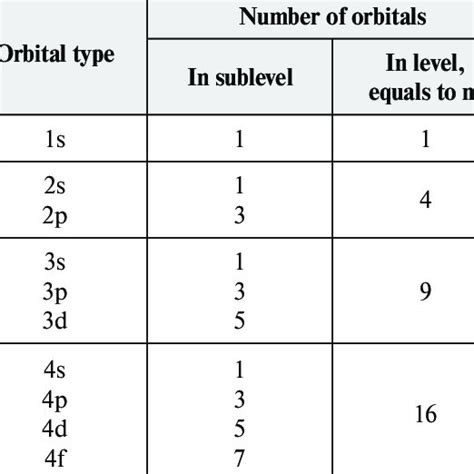
Table of Contents
Maximum Number of Electrons in a d Orbital: A Deep Dive into Atomic Structure
Understanding the maximum number of electrons a d orbital can hold is crucial for grasping fundamental concepts in chemistry and physics. This seemingly simple question opens a door to a fascinating exploration of atomic structure, electron configurations, and the quantum mechanical principles governing the behavior of matter at the atomic level. This article will delve deep into this topic, explaining not just the answer but the underlying reasons and implications.
The Quantum Mechanical Model and Atomic Orbitals
To understand the maximum electron capacity of a d orbital, we need to briefly review the quantum mechanical model of the atom. This model describes electrons not as orbiting particles in specific paths, like planets around the sun (the Bohr model), but as existing in regions of space called atomic orbitals. These orbitals are defined by a set of four quantum numbers:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can take on positive integer values (n = 1, 2, 3...). Higher values of 'n' indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and its angular momentum. It can have integer values from 0 to n-1. Different values of 'l' correspond to different subshells:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can have integer values from -l to +l, including 0. For example:
- For a p orbital (l=1), ml can be -1, 0, or +1, representing three p orbitals (px, py, pz) oriented along the x, y, and z axes.
- For a d orbital (l=2), ml can be -2, -1, 0, +1, +2, representing five d orbitals.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can have only two values: +1/2 (spin up) or -1/2 (spin down). This is crucial for understanding the Pauli Exclusion Principle.
The Pauli Exclusion Principle and Electron Capacity
The Pauli Exclusion Principle is a cornerstone of quantum mechanics. It states that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, with opposite spins.
Unveiling the Mystery: 10 Electrons in d Orbitals
Now, let's connect the dots. Since a d orbital is defined by l = 2, the magnetic quantum number (ml) can have five possible values (-2, -1, 0, +1, +2). This means there are five different d orbitals within a given electron shell (e.g., 3d, 4d, 5d, etc.).
Because each of these five d orbitals can hold a maximum of two electrons (one spin up and one spin down, as per the Pauli Exclusion Principle), the total maximum number of electrons a d subshell can accommodate is 5 orbitals * 2 electrons/orbital = 10 electrons.
Visualizing the d Orbitals
While the shapes of s and p orbitals are relatively straightforward to visualize, the five d orbitals are more complex. They have a variety of shapes, including some with lobes along the axes and others with lobes between the axes. These complex shapes arise from the mathematical solutions to the Schrödinger equation for the d orbitals.
Implications of the Maximum Electron Capacity of d Orbitals
The fact that d orbitals can hold up to ten electrons has significant implications in several areas:
-
Transition Metal Chemistry: Transition metals are characterized by partially filled d orbitals. The variable oxidation states and the rich and diverse chemistry of transition metals are directly linked to the ability of these d orbitals to accommodate varying numbers of electrons. The d electrons participate in bonding, leading to complex coordination compounds and catalytic activities.
-
Spectroscopy: The electronic transitions between different d orbitals in transition metal complexes give rise to characteristic absorption spectra, which are used for identification and analysis. The energy differences between these orbitals determine the colors observed in many transition metal compounds.
-
Material Science: The electronic structure of materials, particularly those containing transition metals, is profoundly influenced by the d orbitals. This has implications for the magnetic properties, electrical conductivity, and catalytic behavior of various materials. For instance, the magnetic properties of many ferromagnetic materials stem from interactions between unpaired d electrons.
-
Catalysis: Many important catalysts, including those used in industrial processes and biological systems, involve transition metals. The ability of the d orbitals to accept and donate electrons facilitates the catalytic processes.
-
Quantum Computing: The complex properties of d orbitals are relevant to the development of quantum computing technologies. The study and manipulation of the quantum states of electrons in these orbitals are key areas of research.
Beyond the Basics: Electron Configuration and Hund's Rule
Understanding the maximum number of electrons in a d orbital is just the beginning. The actual electron configuration of an atom—how electrons fill the orbitals—is governed by several principles, including the Aufbau principle, which dictates that electrons fill orbitals from lowest to highest energy, and Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This rule maximizes the total spin of the electrons.
For example, consider chromium (Cr), which has an atomic number of 24. A simplistic Aufbau principle approach might suggest a configuration of [Ar] 3d<sup>4</sup> 4s<sup>2</sup>. However, Hund's rule and the stability gained by having a half-filled d subshell lead to the actual electron configuration of [Ar] 3d<sup>5</sup> 4s<sup>1</sup>. This highlights the importance of considering all the rules governing electron configurations.
Advanced Concepts and Further Exploration
The topic of electron configuration and atomic orbitals can extend into much more advanced areas of quantum chemistry. Concepts such as:
-
Hybridization: The mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. This is particularly important in understanding molecular geometry and bonding.
-
Molecular Orbital Theory: A framework that describes bonding in molecules by considering the combination of atomic orbitals to form molecular orbitals.
-
Ligand Field Theory: A model that describes the bonding and electronic structure of transition metal complexes, taking into account the interactions between the metal d orbitals and the ligands surrounding the metal ion.
These areas provide deeper insights into the behavior of electrons in atoms and molecules, building upon the fundamental understanding of the maximum electron capacity of the d orbital.
Conclusion: A Foundation for Chemical Understanding
Understanding the maximum number of electrons that a d orbital can hold – ten electrons – forms a crucial foundation for understanding a vast range of chemical and physical phenomena. This knowledge allows us to interpret the properties of transition metals, predict the behavior of compounds, and explore advanced concepts in quantum chemistry and material science. The interplay between the quantum mechanical principles, electron configuration rules, and the unique characteristics of the d orbitals reveals the intricacies of the atomic world and opens doors to various scientific and technological advancements. This knowledge, however, represents only the beginning of a deeper and more comprehensive study of atomic structure and the properties of matter.
Latest Posts
Latest Posts
-
What Percent Is 70 Of 280
Mar 26, 2025
-
Where Does The Krebs Cycle Take Place In The Mitochondria
Mar 26, 2025
-
Correctly Label The Following Parts Of The Testis
Mar 26, 2025
-
Which Of The Following Equations Is True
Mar 26, 2025
-
The Molar Mass Of Cuso4 5h2o Is 249
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Maximum Number Of Electrons In D Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
