The Gas Which Turns Lime Water Milky Is
News Leon
Mar 23, 2025 · 6 min read
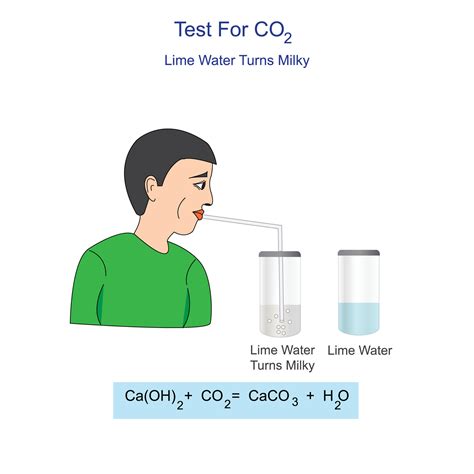
Table of Contents
The Gas That Turns Limewater Milky: A Deep Dive into Carbon Dioxide
The simple observation of limewater turning milky is a classic chemistry experiment, a cornerstone of understanding the properties of gases. But what's really happening at a molecular level? This article delves deep into the science behind this phenomenon, exploring the gas responsible – carbon dioxide (CO2) – its properties, its role in various processes, and its broader significance in our environment and everyday lives.
Understanding Limewater and its Reaction with CO2
Limewater, also known as calcium hydroxide solution (Ca(OH)₂), is a clear, colorless solution of calcium hydroxide in water. Its seemingly simple composition belies its reactivity, particularly with carbon dioxide. The milky appearance that results from the interaction between limewater and CO2 is a visual manifestation of a chemical reaction, specifically a precipitation reaction.
The Chemical Reaction: A Closer Look
When carbon dioxide gas is bubbled through limewater, it reacts with the calcium hydroxide to form calcium carbonate (CaCO₃) and water (H₂O). This reaction can be represented by the following balanced chemical equation:
Ca(OH)₂(aq) + CO₂(g) → CaCO₃(s) + H₂O(l)
The key here is the formation of calcium carbonate (CaCO₃), an insoluble white solid. This solid precipitates out of the solution, causing the limewater to become cloudy or milky. The change in appearance is a direct result of the insoluble calcium carbonate particles scattering light.
Beyond the Milkiness: Understanding the Precipitation
The precipitation of calcium carbonate is a crucial aspect of this reaction. Solubility, the ability of a substance to dissolve in a solvent, is a critical factor. Calcium carbonate has very low solubility in water, meaning it doesn't readily dissolve. When it's formed in the reaction, it quickly exceeds its solubility limit, leading to the formation of solid particles that remain suspended in the limewater, creating the characteristic milky appearance.
The Properties of Carbon Dioxide: A Key Player
Carbon dioxide's role in turning limewater milky highlights its fundamental chemical properties. It's a colorless, odorless gas that's slightly denser than air. These properties, along with its reactivity, make it a significant component of Earth's atmosphere and many natural and industrial processes.
Carbon Dioxide's Reactivity: More Than Just Limewater
While its reaction with limewater is a well-known demonstration, CO2 exhibits a range of other chemical reactions. Its acidic nature allows it to react with bases like calcium hydroxide. It can also react with other compounds to form carbonates, bicarbonates, and even more complex structures.
Sources of Carbon Dioxide: Natural and Anthropogenic
Carbon dioxide is naturally present in the atmosphere as part of the Earth's carbon cycle. Volcanic eruptions, respiration in living organisms (both plants and animals), and the decomposition of organic matter all contribute to its atmospheric concentration. However, human activities, particularly the burning of fossil fuels (coal, oil, and natural gas) for energy, deforestation, and industrial processes, have significantly increased atmospheric CO2 levels, leading to global climate change.
The Significance of the Limewater Test: Applications and Implications
The limewater test for carbon dioxide is not just a laboratory curiosity. It has various practical applications and implications:
Identifying Carbon Dioxide: A Simple and Effective Test
The simplicity and reliability of the limewater test make it a valuable tool for identifying the presence of carbon dioxide. This is frequently utilized in schools, laboratories, and even in some industrial settings to confirm the presence of CO2.
Understanding Respiration: A Biological Perspective
The limewater test can be used to demonstrate the process of respiration in living organisms. By collecting the gas exhaled by humans or animals and bubbling it through limewater, the formation of calcium carbonate clearly shows that carbon dioxide is a byproduct of respiration.
Monitoring Industrial Processes: Quality Control and Safety
In industries that produce or utilize carbon dioxide, limewater tests can play a role in quality control and safety monitoring. By detecting leaks or measuring CO2 concentrations, this simple test can contribute to a safe and efficient operation.
Studying the Carbon Cycle: A Global Perspective
The reaction between limewater and carbon dioxide provides a visual representation of a key component of the Earth's carbon cycle. Understanding this cycle, including the sources, sinks, and fluxes of carbon dioxide, is crucial for addressing climate change.
Environmental Impact of CO2: A Growing Concern
The increasing atmospheric concentration of CO2, largely due to human activities, is causing significant environmental changes. These changes have far-reaching consequences, impacting various ecosystems and the climate as a whole.
Greenhouse Effect and Global Warming: The Interplay of CO2
CO2 is a potent greenhouse gas, meaning it traps heat in the Earth's atmosphere. This "greenhouse effect" is a natural process that keeps the planet warm enough to support life. However, the increase in atmospheric CO2 concentration has amplified this effect, leading to global warming and climate change.
Ocean Acidification: A Hidden Threat
CO2 dissolves in seawater, reacting with water molecules to form carbonic acid (H₂CO₃). This process lowers the pH of the ocean, a phenomenon known as ocean acidification. Ocean acidification has significant consequences for marine life, particularly organisms that build shells and skeletons from calcium carbonate, such as corals and shellfish. These organisms struggle to build and maintain their structures in more acidic waters.
Climate Change Impacts: A Wide Range of Effects
The consequences of climate change are far-reaching and diverse. Rising global temperatures lead to sea-level rise, more frequent and intense extreme weather events (such as heat waves, droughts, floods, and storms), changes in precipitation patterns, and disruptions to ecosystems. These changes impact human societies and economies in many ways, threatening food security, water resources, human health, and infrastructure.
Mitigating CO2 Emissions: A Collective Responsibility
Addressing the environmental challenges posed by CO2 requires a global effort to mitigate its emissions and adapt to the changing climate. Various strategies are being pursued to reduce CO2 emissions:
Transition to Renewable Energy Sources: A Crucial Step
Shifting from fossil fuels to renewable energy sources, such as solar, wind, hydro, and geothermal power, is essential for reducing CO2 emissions. This transition involves technological advancements, policy changes, and investments in renewable energy infrastructure.
Improving Energy Efficiency: Reducing Consumption
Improving energy efficiency in buildings, transportation, and industrial processes is another critical strategy. This involves using more energy-efficient technologies, adopting sustainable building practices, and promoting behavioral changes to reduce energy consumption.
Carbon Capture and Storage: Innovative Technologies
Carbon capture and storage (CCS) technologies capture CO2 emissions from power plants and industrial facilities and store them underground, preventing them from entering the atmosphere. While still under development, CCS technologies offer potential for significantly reducing CO2 emissions from large point sources.
Afforestation and Reforestation: Nature-Based Solutions
Planting trees (afforestation and reforestation) helps absorb CO2 from the atmosphere through photosynthesis. This natural process provides a valuable tool for mitigating climate change and enhancing biodiversity.
Conclusion: The Humble Limewater Test and the Global Challenge
The simple observation of limewater turning milky upon exposure to carbon dioxide reveals a fundamental chemical reaction with significant implications. While seemingly straightforward, this experiment serves as a gateway to understanding the properties of CO2, its crucial role in various processes, and the urgent need to address the environmental challenges posed by its increasing atmospheric concentration. The milky appearance of the limewater is a potent visual reminder of the unseen impact of this gas on our planet and the collective responsibility to mitigate its effects. The journey from a simple chemistry experiment to comprehending the global implications of CO2 underscores the interconnectedness of scientific understanding and environmental stewardship. The future of our planet depends on our ability to translate this knowledge into effective action.
Latest Posts
Latest Posts
-
State Whether The Following Statements Are True Or False
Mar 24, 2025
-
In Which Stage Of Meiosis Crossing Over Takes Place
Mar 24, 2025
-
Which Of The Following Is Not A Major Nutrient
Mar 24, 2025
-
The Figure Gives The One Dimensional Potential Energy
Mar 24, 2025
-
Saying The Opposite Of What You Mean
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about The Gas Which Turns Lime Water Milky Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
